true
Support
Customer Care & Ordering
866 979 9408, (905 288 6000 non toll free)
Contact Us
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.


- Overview
- Best Practices
- Products & Accessories
- EIFU & Resources
BD Nexiva™ Closed IV Catheter System is not just a catheter. BD Nexiva™ Closed IV Catheter System is an all-in-one PIVC, combining four individual devices in one: IV catheter, extension set, stabilization device, and needle-free connector(s). It is clinically demonstrated to have longer dwell times – dwelling up to 144 hours – and reduced complications versus an open system1. Discover more about the BD NexivaTM Closed IV Catheter System and how it can support your clinical practice here.
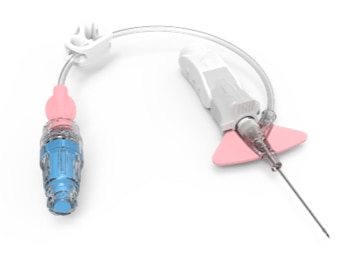
Built-in stabilization platform and integrated extension set
Designed to reduce manipulation and movement at the site. Have been shown to reduce dislodgment by 84% and mechanical phlebitis.1,2
98% reduced blood exposure during insertion due to the pre-assembled system.2
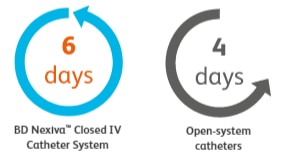
Dwells longer
Median dwell time for BD NexivaTM Closed IV Catheters versus the open-system catheters studied in a randomized trial of PIVCs in place for more than 24 hours.1

BD Vialon™ catheter material
Proprietary BD Vialon™ Catheter Material softens, enabling longer dwell time and reducing the chance of phlebitis up to 69%.3
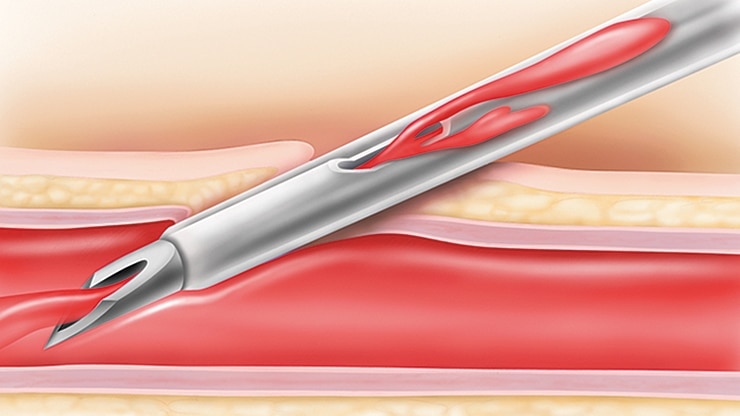
BD Instaflash™ needle technology
Provides quick blood visualization that may help improve insertion success and therefore reduce insertion attempts.
BD Nexiva™: Traditional vs closed IV catheter insertion
Canadian Vascular Access & Infusion Therapy Guidelines
Recommend using extension set (integrated preferred) on PVAD (to reduce manipulations and avoid dislodgement), and using suture-less securement (including engineered securement devices) to limit movement of VAD. Method of securement can include an integrated stabilization feature on PVAD.
Recommend not removing VAD based solely on length of dwell time, as optimum dwell time is unknown.6
Centers for Disease Control and Prevention
Catheter stabilization is recognized as an intervention to decrease the risk for phlebitis, catheter migration and dislodgement and may be advantageous in preventing catheter-related bloodstream infections (CRBSIs).3
Infusion Therapy Standards of Practice
Recommend limiting the use of add-on devices to reduce the potential for contamination, additional manipulation, and disconnection.5
Increased clinician safety
98% reduced blood exposure during insertion due to the BD Nexiva IV catheter preassembled system.2*
Increased catheter stabilization
Clinically demonstrated to reduce accidental dislodgement,2‡ meeting Infusion Nursing Society standards4 and CDC guidelines5 for catheter stabilization.
Reduced rate of complications
In a clinical study, results demonstrated a significant reduction in the rate of phlebitis (grade 2 or higher), PIVC-related complications, and infiltration in the closed system versus the open system group.1
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
true
false
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114(10):845-854.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2016:39(1S).
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.
- Canadian Vascular Access Association. (2019). Canadian Vascular Access and Infusion Therapy Guidelines. Pembroke, ON: Pappin Communications.
true

