Indications
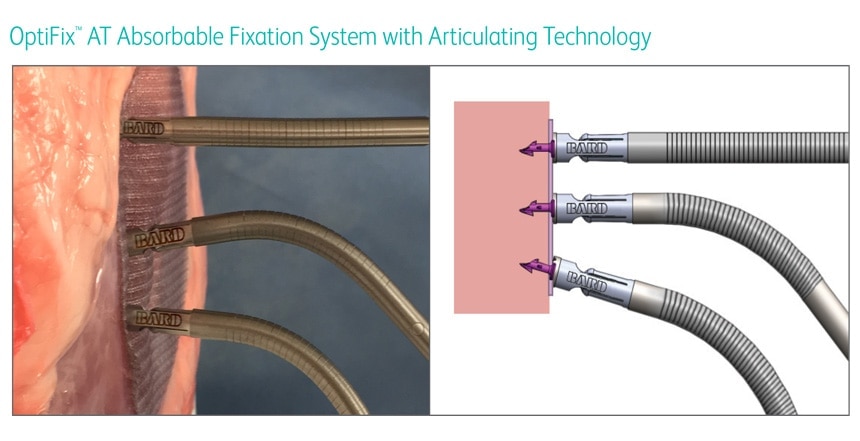
OptiFix™ AT Absorbable Fixation System with Articulating Technology is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to:
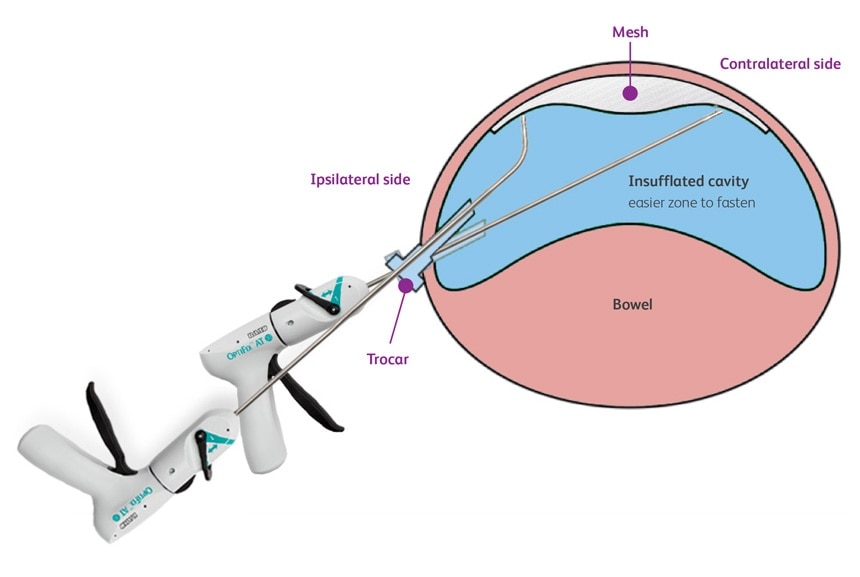
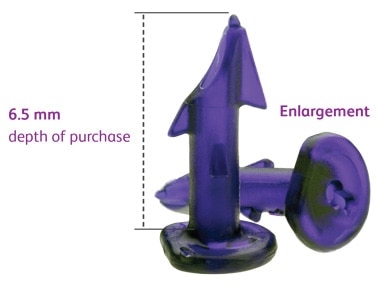
- Fixation in the close vicinity of underlying structures such as nerves, vessels, viscera, cartilage or bone. Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera, cartilage or bone. For reference, the length of the fastener below the fastener head is 6.5 mm, and the fastener head is another 0.6 mm (total 7.1 mm).
- Fixation of or in proximity to tissues that have a direct anatomic relationship to major vascular structures. This would include the deployment of fasteners in the diaphragm in the vicinity of the pericardium, aorta, or inferior vena cava during diaphragmatic hernia repair.
- Fixation of vascular or neural structures, viscera, cartilage or bone.
- Situations with insufficient in-growth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is absorbed.
Warnings
Remove the yellow clip prior to use of device. Do not use device if the yellow clip is damaged or not in place. The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use. After use, the OptiFix™ AT Absorbable Fixation System with Articulating Technology may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Adverse Reactions
Potential complications with the use of any prosthesis may include, but are not limited to hemorrhage; pain, erosion, edema and erythema at wound site; allergic reaction to poly(L-lactide-co-glycolide); septicemia/infection; hernia recurrence/wound dehiscence.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use.
1. Preclinical data on file at BD. Results may not correlate to performance in humans.