The Criticore™ Monitor is a fluid output and core temperature monitor for use on care patients in the ER, OR, and the ICU.
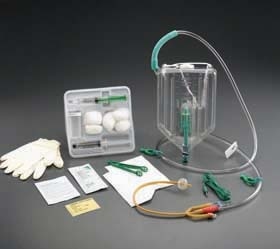
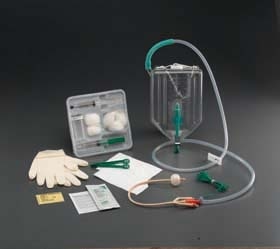
The Criticore™ Infection Control Foley Tray is for use on all critical care patients who are at risk for developing urinary tract infection. This tray contains a 2000ml Criticore™ Collection Container pre-connected with the BARD® patented Tamper-Evident Seal to a Bardex™ I.C. anti-infective Temperature Sensing Latex Foley Catheter, or a Lubri-Sil™ I.C. antimicrobial Temperature Sensing Foley catheter, each with a 5cc balloon. Both the Bardex™ I.C. and Lubri-Sil™ I.C. Foley catheters feature the Bacti-Guard™* silver alloy coating and BD hydrogel and are compatible with other temperature monitors that accept 400-Series temperature probes.
Additionally, the Complete Care™ System incorporates a bacteriostatic agent in the drain tube and drain bag material to repel common bacteria and yeasts. With BD's 6 Point Prevention strategy, the Complete Care™ System offers infection control technology built into each component of the urine drainage system.