Contact Support
The portable, easy-to-use BD Veritor™ System provides reliable COVID-19 (SARS-CoV-2) results in 15 minutes
The BD Veritor™ System for Rapid Detection of SARS-CoV-2 is a chromatographic immunoassay for the direct and qualitative detection of SARS-CoV-2 antigens in nasal swabs from patients with signs and symptoms who are suspected of COVID-19.
![]()
Displays easy-to-read digital results for SARS-CoV-2 in 15 minutes.
![]()
Easy operation and 1-button functionality may help reduce the potential for procedural errors.
![]()
Adapts easily to your workflow with 2 operational modes: Walk Away and Analyze Now.
Easy to use. Flexible workflow. Fast, traceable results.
Meet patient and staff needs with simple, timely point-of-care testing.
Simplify the testing process
Achieve fast, reliable results
Provide workflow efficiency
Provide result traceability
*in comparison to visually read tests
These are the steps for sample preparation, including the process for each workflow mode of the BD Veritor™ Plus System.
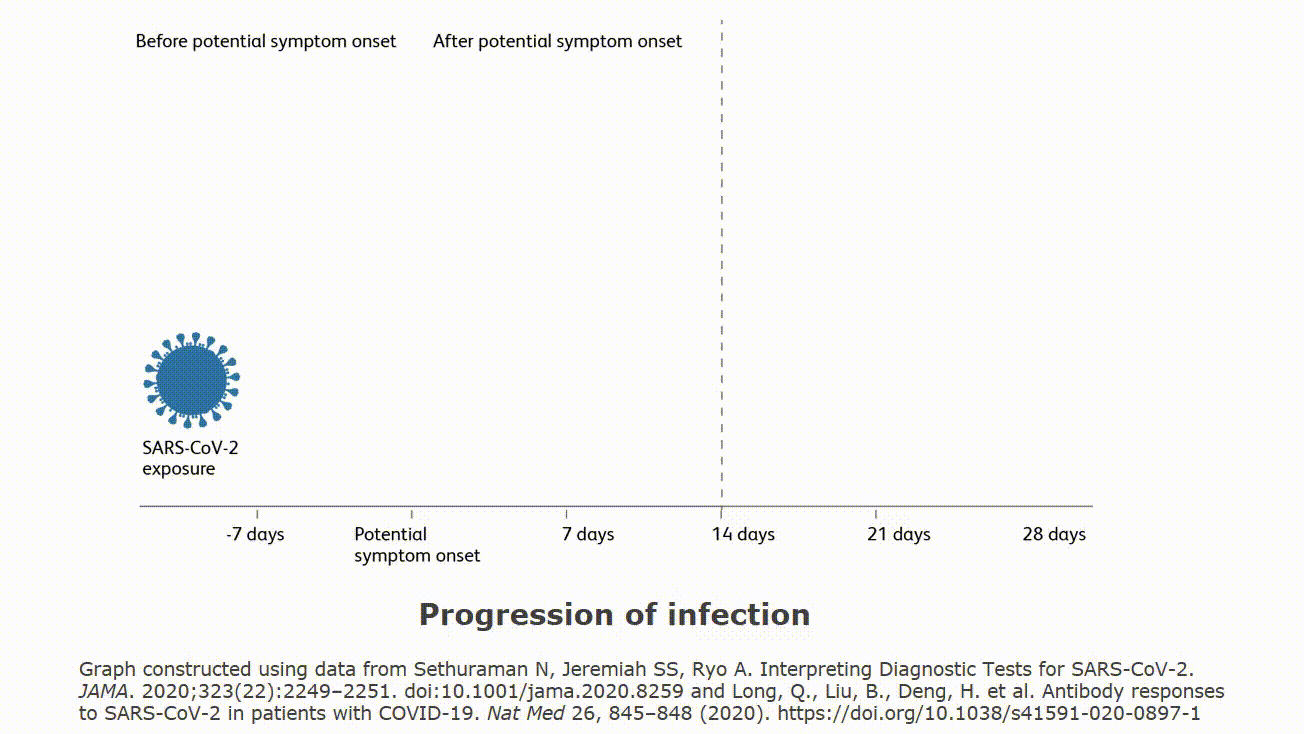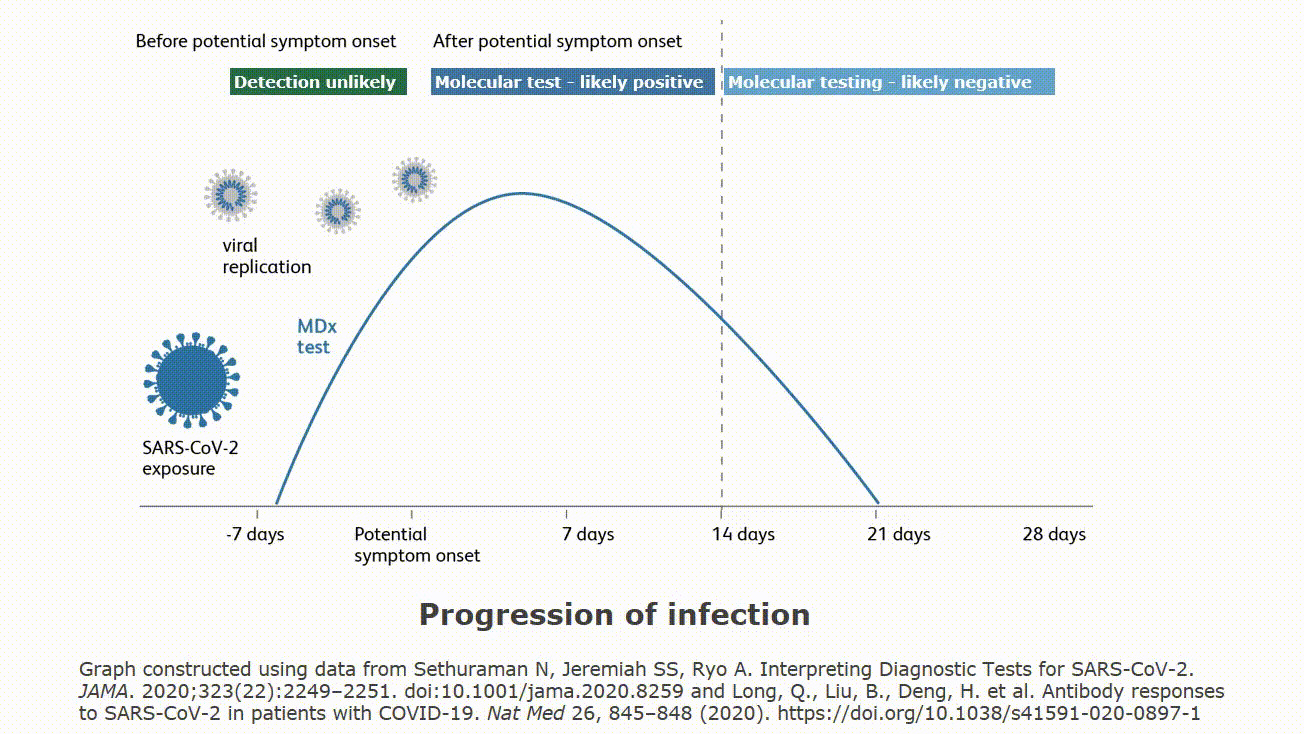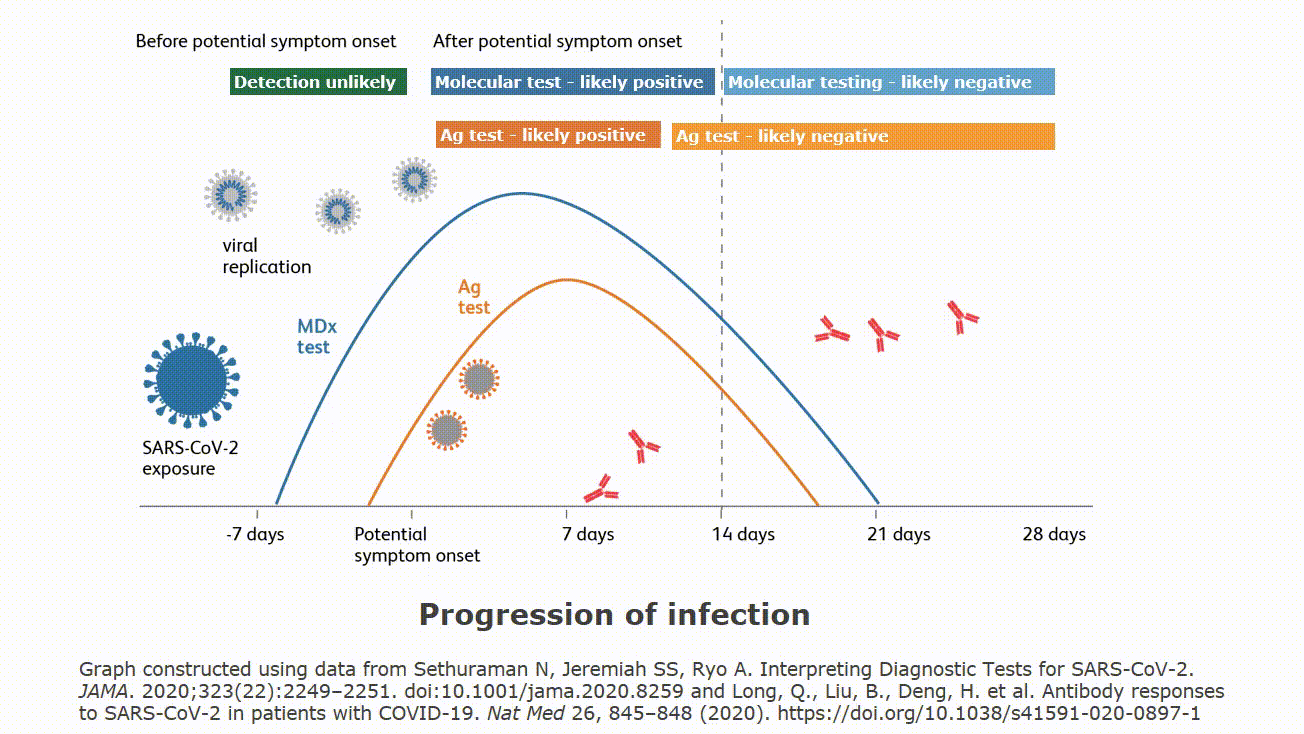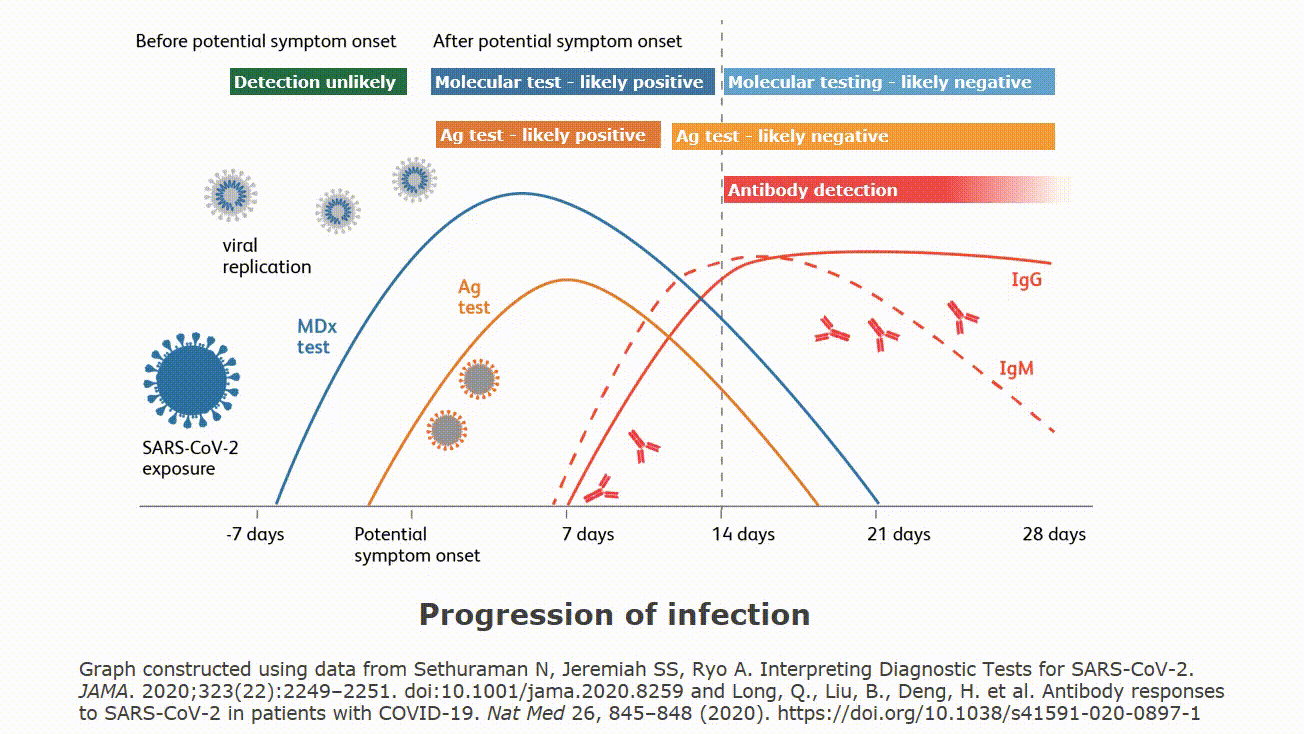
It is important to understand how local disease prevalence rate and test performance characteristics interact to influence the proportion of false positive test results compared to all positive results. The infographic below demonstrates how very low, low, medium and high prevalence rates make a difference. It is important to note that your institution’s specific prevalence rates can be used to calculate the potential in your facility. Understanding that false positive test results are a possibility is important. Please follow your institution and local guidance for both addressing a patient with a positive test result. Guidance may include precautionary isolation procedures and a second mode of testing for confirmation.
Please Note: The above data represents clinical performance data taken from Study 2 in the package insert. Performance established with patients reporting two or more self-reported symptoms.
Even the most highly-accurate tests can produce false positive results. False positives are expected for all diagnostic tests, involving all detection technologies: PCR, antigen assays, etc. In the case of antigen tests (such as BD Veritor™ Plus System), test results could be influenced by a number of factors:
Understanding the specifics of test performance can be complex. Below is the list of test performance terminology which may help you to better understand.
Learn more about prevalence rates and testing outcomes: https://www.cdc.gov/coronavirus/2019-ncov/downloads/lab/fda-calculator.pdf
Learn more about interim guidance for rapid antigen testing for SARS-CoV-2: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html#
| Rapid Antigen Testing for SARS-CoV-2 | |
|---|---|
| Catalog no. | Description |
| 256089 | BD Veritor System for Rapid Detection of SARS-CoV-2 |
| BD Veritor System Analyzer | |
|---|---|
| Catalog no. | Description |
| 256066 | BD Veritor Plus Analyzer |
The comprehensive eLearning platform for the BD Veritor™ Plus System streamlines and simplifies training and tracking (local regulation or accreditation efforts), enabling you to quickly train staff and implement point-of-care testing.
BD eLearning platform provides you with:

Simplify SARS-CoV-2 testing with BD Veritor™ Plus - Brochure


BD Veritor™ Plus System eLearning - Brochure

BD Veritor™ Plus SARS-CoV-2 Assay instructions for use (IFU)

Analyze Now mode batch testing guide – BD Veritor™
Quick reference guide - BD Veritor™

Nasal Swab Quick reference guide - BD Veritor™

Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.