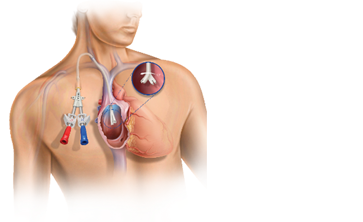
Healthcare providers rely on us for comprehensive solutions across discovery, diagnostics and delivery of care at critical moments throughout their patients’ lives.
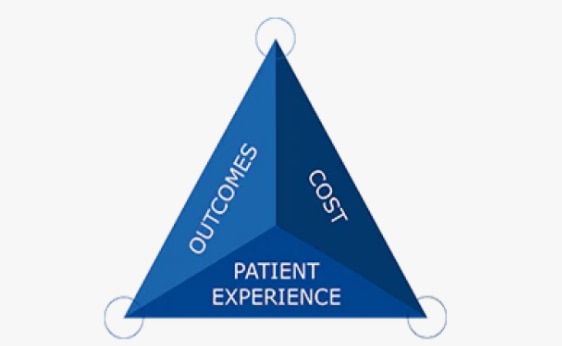
By leveraging our global network of cutting-edge science and technology, powerful partnerships and the brightest minds in med tech, we’re helping healthcare professionals and organizations achieve better outcomes and patient experiences, while optimizing care delivery.
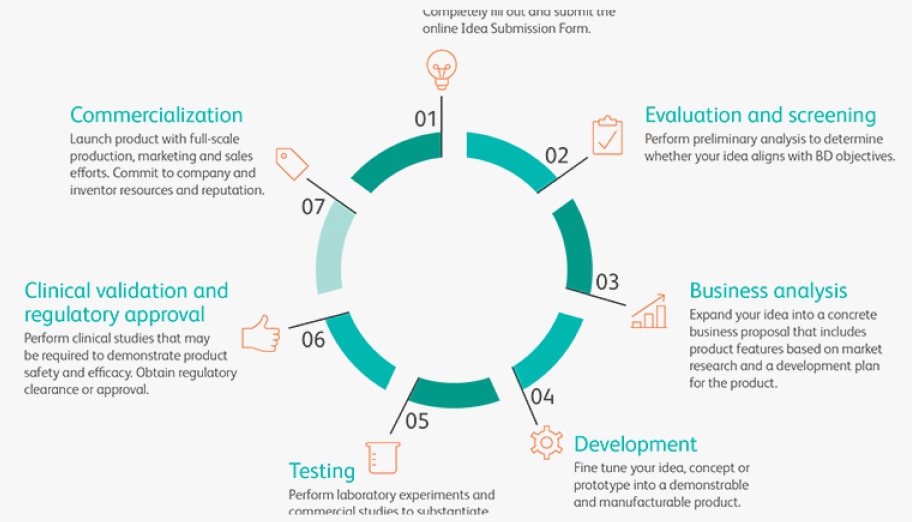
As we continuously innovate our category-leading core platforms, we're also focused on three irreversible trends driving the future of healthcare.