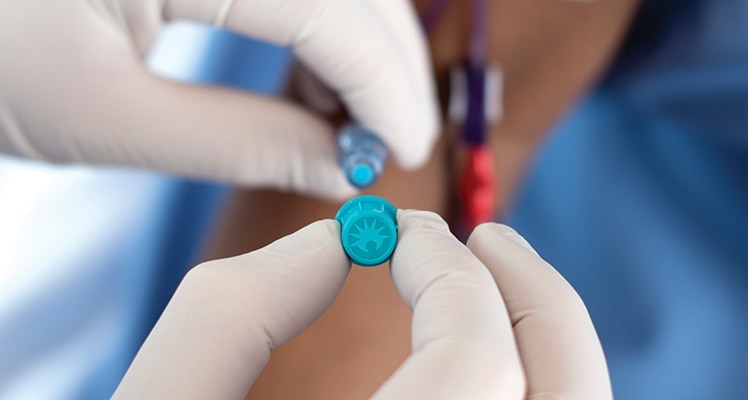
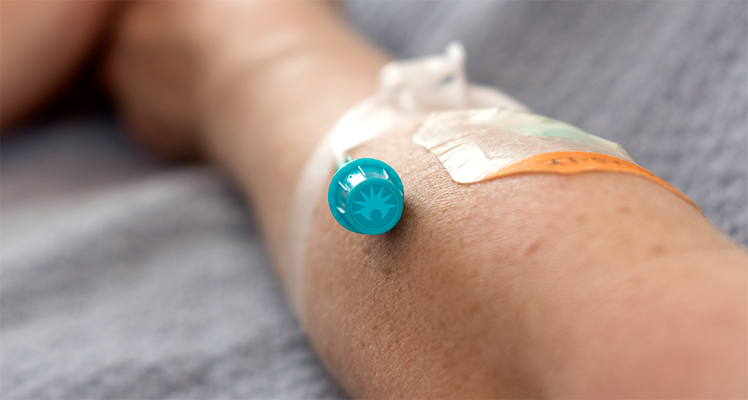
The BD PureHub™ Disinfecting Cap acts as a physical barrier between line accesses and is indicated as a disinfecting device for swabbable needle-free luer connectors prior to I.V. access.
BD Purehub™ Disinfecting Caps are part of the full BD® Vascular Access Management (VAM) portfolio designed to help reduce catheter related complications.
The use of disinfecting caps is supported by the 2014 Compendium of Prevention Recommendations from the Society for Healthcare Epidemiology of America (SHEA)1, The Joint Commission Central Line-Associated Bloodstream Infections (CLABSI) Toolkit: Valve Disinfection guidance2, the 2021 Infusion Nursing Standards of Practice3 (INS) and the Royal College of Nursing Infusion Therapy Standards4.