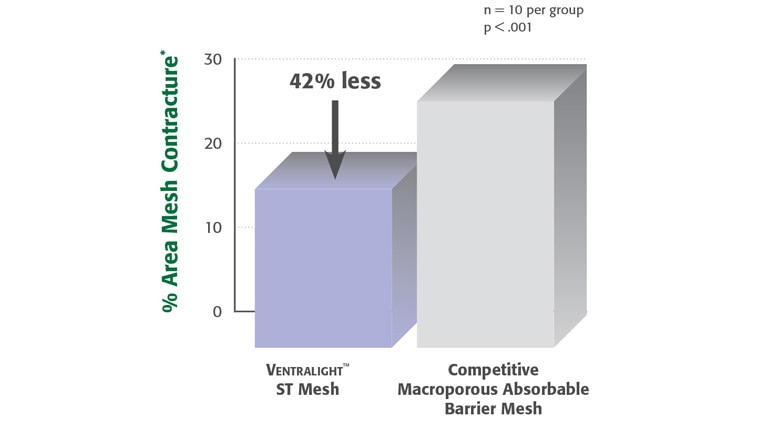
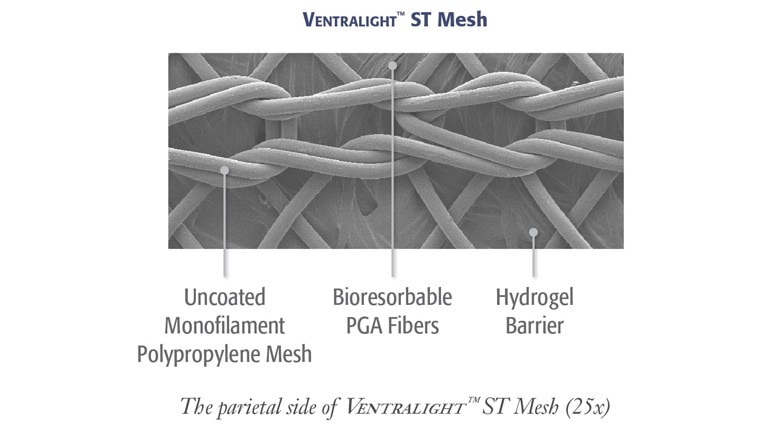
*Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies in the repair of ventral, incisional, and umbilical hernias.
Contraindications
Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by the use of such mesh materials.
The use of this mesh has not been studied in breastfeeding or pregnant women.
Do not use this mesh for the reconstruction of cardiovascular defects.
Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
Warnings
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard with care to prevent risk of transmission of viral infections.
To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
This mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
This mesh has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness, or death of the patient or end user.
This mesh should be used once the exterior foil pouch has been opened. Do not store for later use. Unused portions of the mesh should be discarded.
Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
This mesh is not for the use of treatment of stress urinary incontinence.
Precautions
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this mesh.
The safety and effectiveness of Ventralight™ ST Mesh has not been evaluated in clinical studies in the presence of malignancies in the abdominopelvic cavity.
Adverse Reactions.
Possible complications may include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect.