BD® ChloraPrep™ Patient Preoperative Skin Preparation
Industry-leading 7-day antimicrobial persistence1
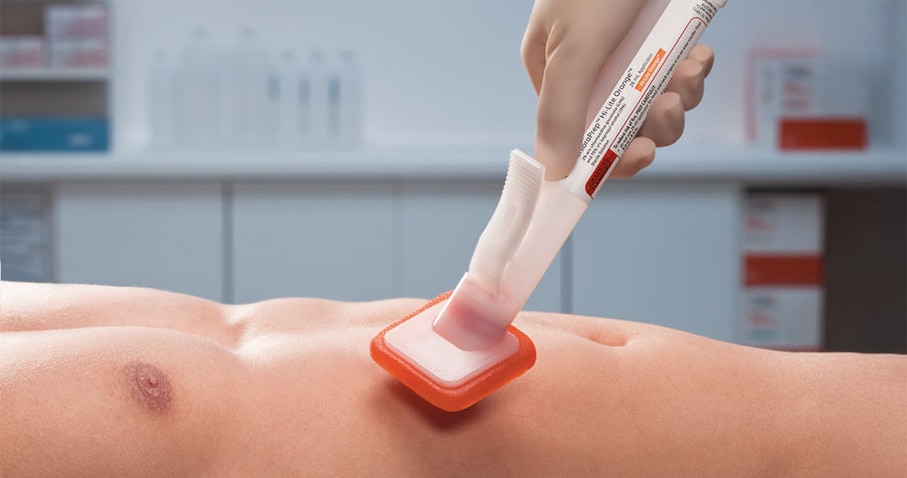
Prep and protect with BD ChloraPrep™ skin prep
BD ChloraPrep™ Patient Preoperative Skin Preparation with Sterile Solution delivers standardized, powerful, persistent antimicrobial protection for at least 7 days. Supported by more than 60 clinical studies, it has been the trusted choice of healthcare providers (HCPs) for over 25 years.
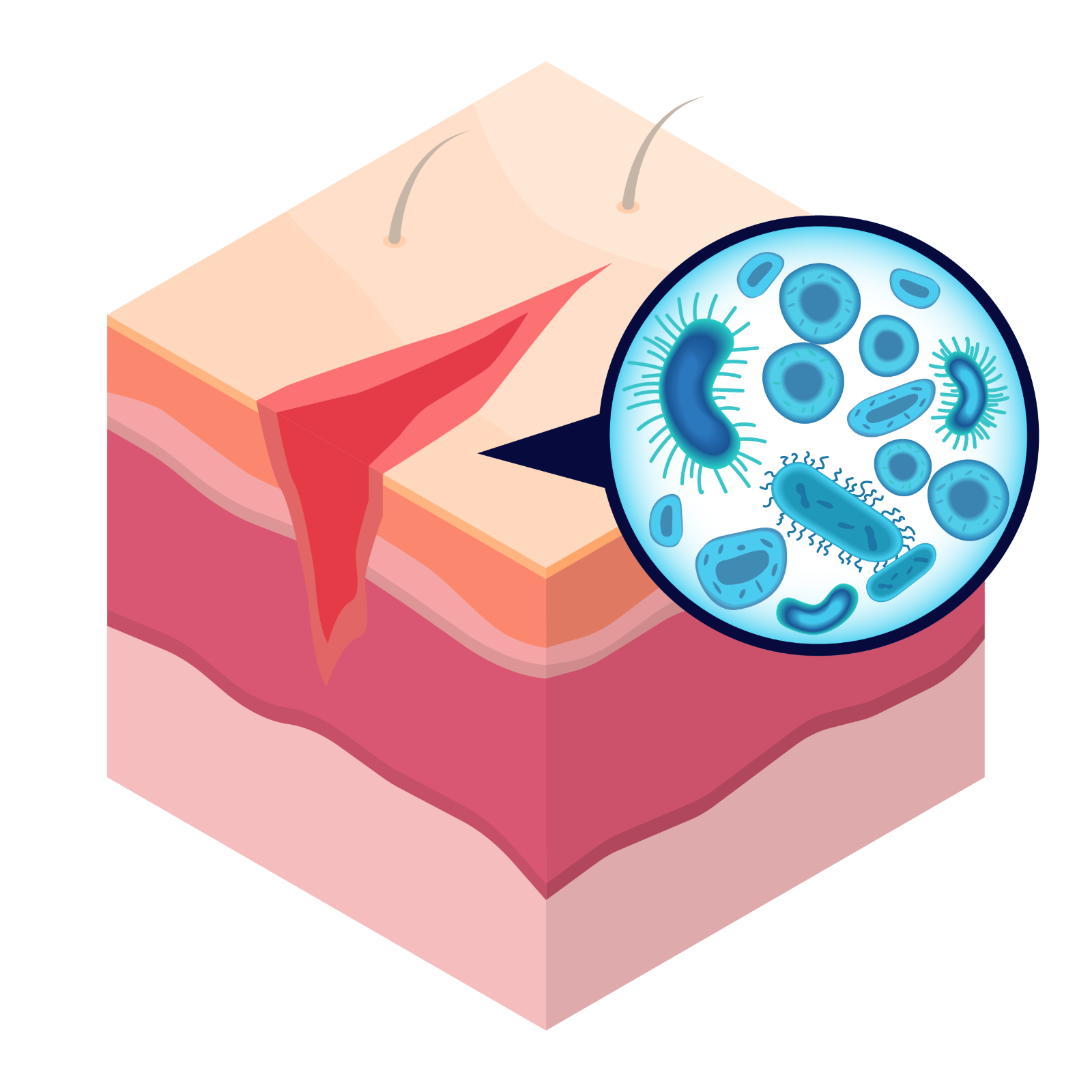
Preoperative skin antisepsis is a critical step in reducing bacteria on the skin that can lead to infection.2 When it comes to choosing the right antiseptic for each patient, confidence matters. That’s why BD ChloraPrep™ skin prep is the trusted choice for lasting protection.
Indication for Use: Use or the preparation of the patient’s skin prior to surgery. Helps to reduce bacteria that potentially can cause skin infection.
Does your antiseptic meet the highest standards?
Clinically Proven
Backed by 60+ clinical studies demonstrating scientifically proven results1
Long-lasting Protection
Offers persistent antimicrobial activity for at least 7 days
Sterile Solution
The most comprehensive CHG/IPA skin prep portfolio with fully sterile applicators3
Hi-Lite Orange™ Tint
Helps mark the prep area and reduces the risk of clinical misdiagnosis based on skin coloring during post-op care
USP® Compliance
Sets quality, purity, strength, and consistency of the product
BD ChloraPrep™ is a broad-spectrum, rapid-acting patient preoperative skin preparation with persistent antimicrobial activity for at least 7 days.
As the industry leader with more than 7 billion applicators sold,1 BD stands unmatched in delivering a comprehensive portfolio of applicator-based skin prep solutions.
Available in 1 mL, Frepp™ 1.5 mL, 3 mL, 10.5 mL, and 26 mL sizes
Backed by more than 60 peer-reviewed clinical studies, BD ChloraPrep™ preoperative skin antiseptic demonstrates category leadership through clinical evidence
The science behind the selection—factors to consider when choosing a skin antiseptic include:
The power of peer review
Randomized controlled trials (RCTs) provide rigorous validation of antiseptic efficacy in clinical practice4
Evidence hierarchy
Meta-analyses and systematic reviews of RCTs represent the highest level of clinical evidence, offering HCPs with the most reliable data for decision-making5
Number of peer-reviewed studies by level of evidence6
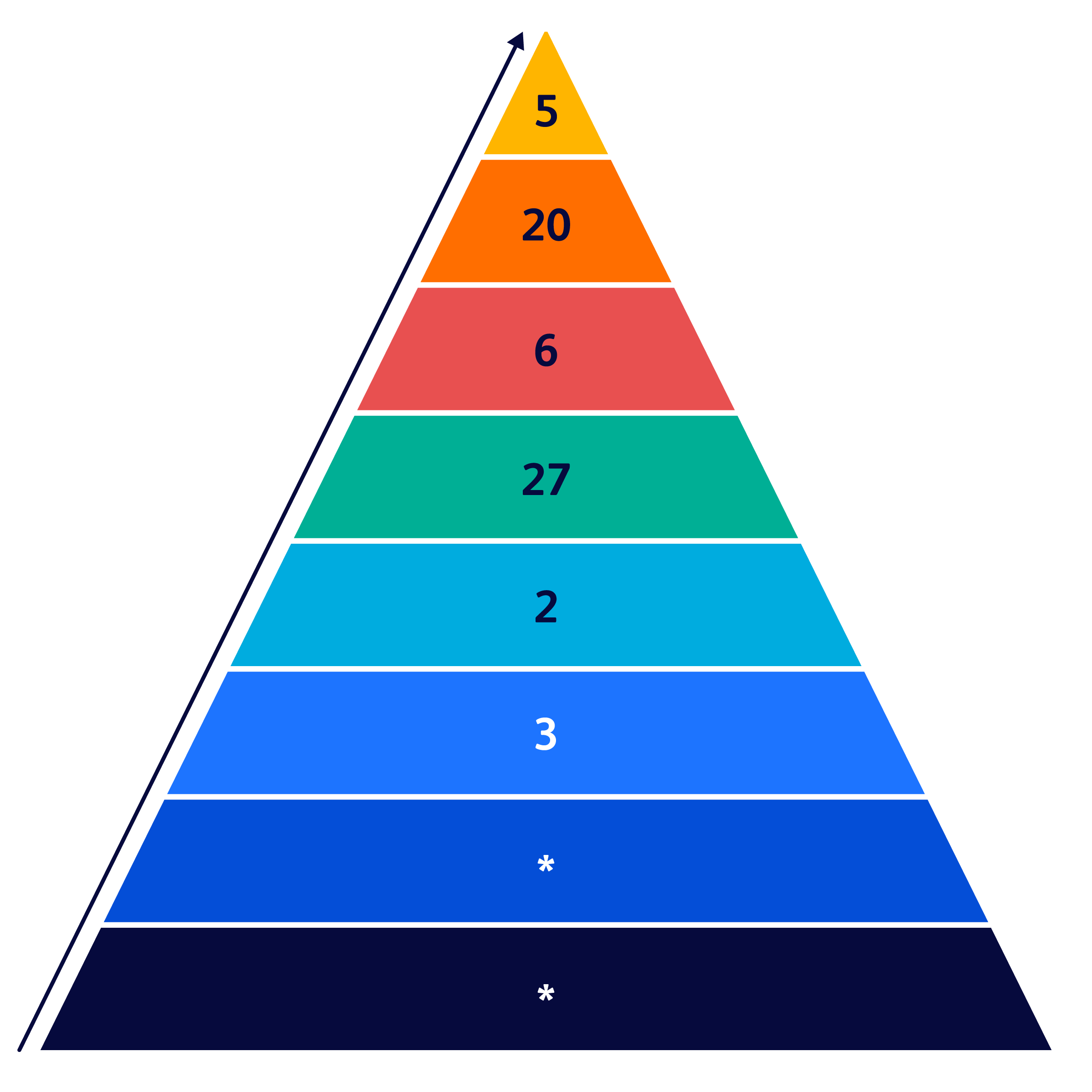
*Data on file.
Hierarchy of evidence is assigned to studies based on the methodological quality of their design, validity, and applicability to patient care. These decisions determine the "grade" (or strength) of recommendation. The more rigorous the standards, the lower the potential for bias and the higher the quality of evidence.
Post-op Day 7: Is your antiseptic still active?
When it comes to preoperative skin antiseptics, not all solutions are created equal.
What is persistence? Antimicrobial persistence is defined as post-treatment microbial counts that are less than or equal to pre-treatment counts.8
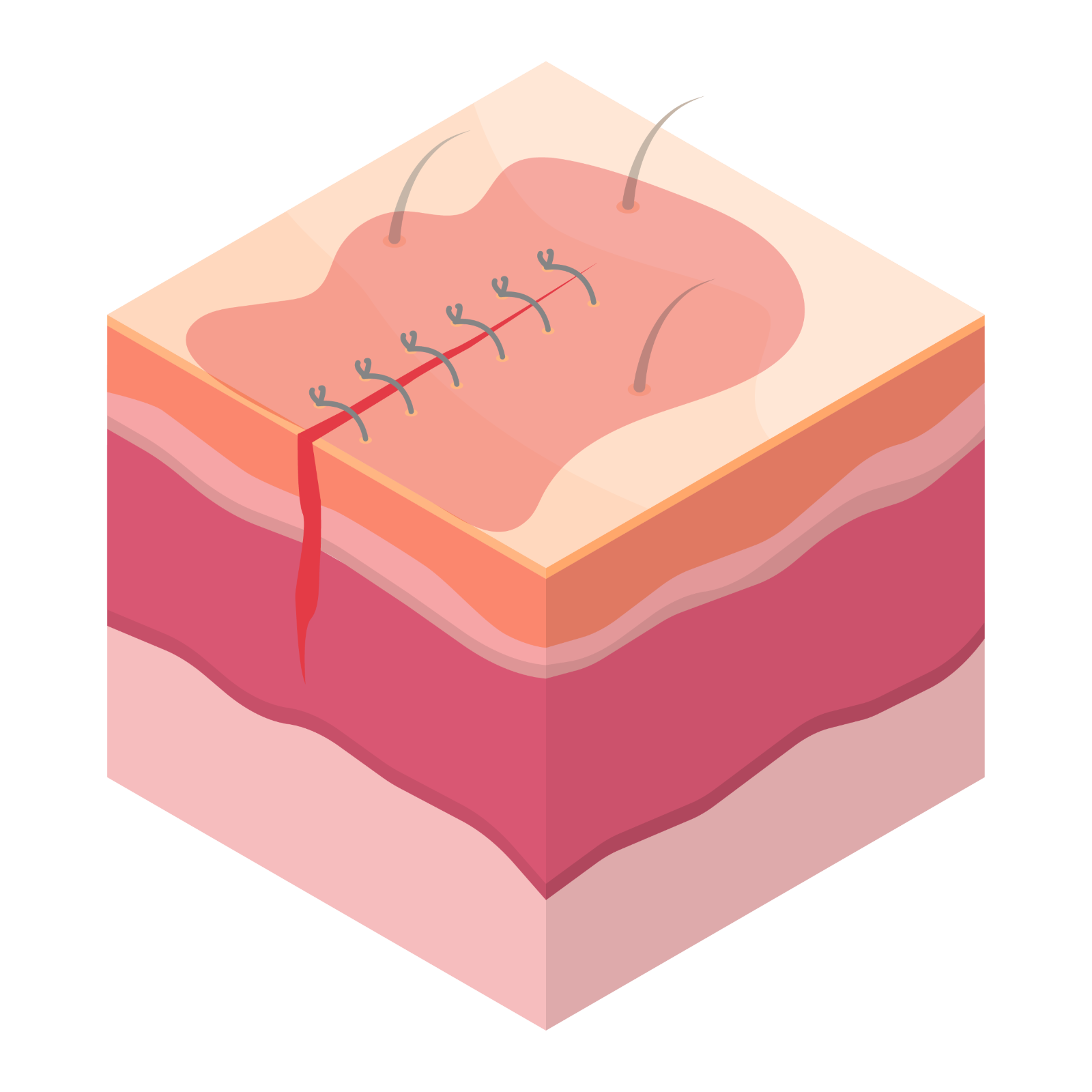
Healing takes time: Re-epithelialization can take up to 2 weeks.9
From incision to re-epithelization, using a skin antiseptic with persistence can help minimize the number of microbes that enter the wound during this time period.10
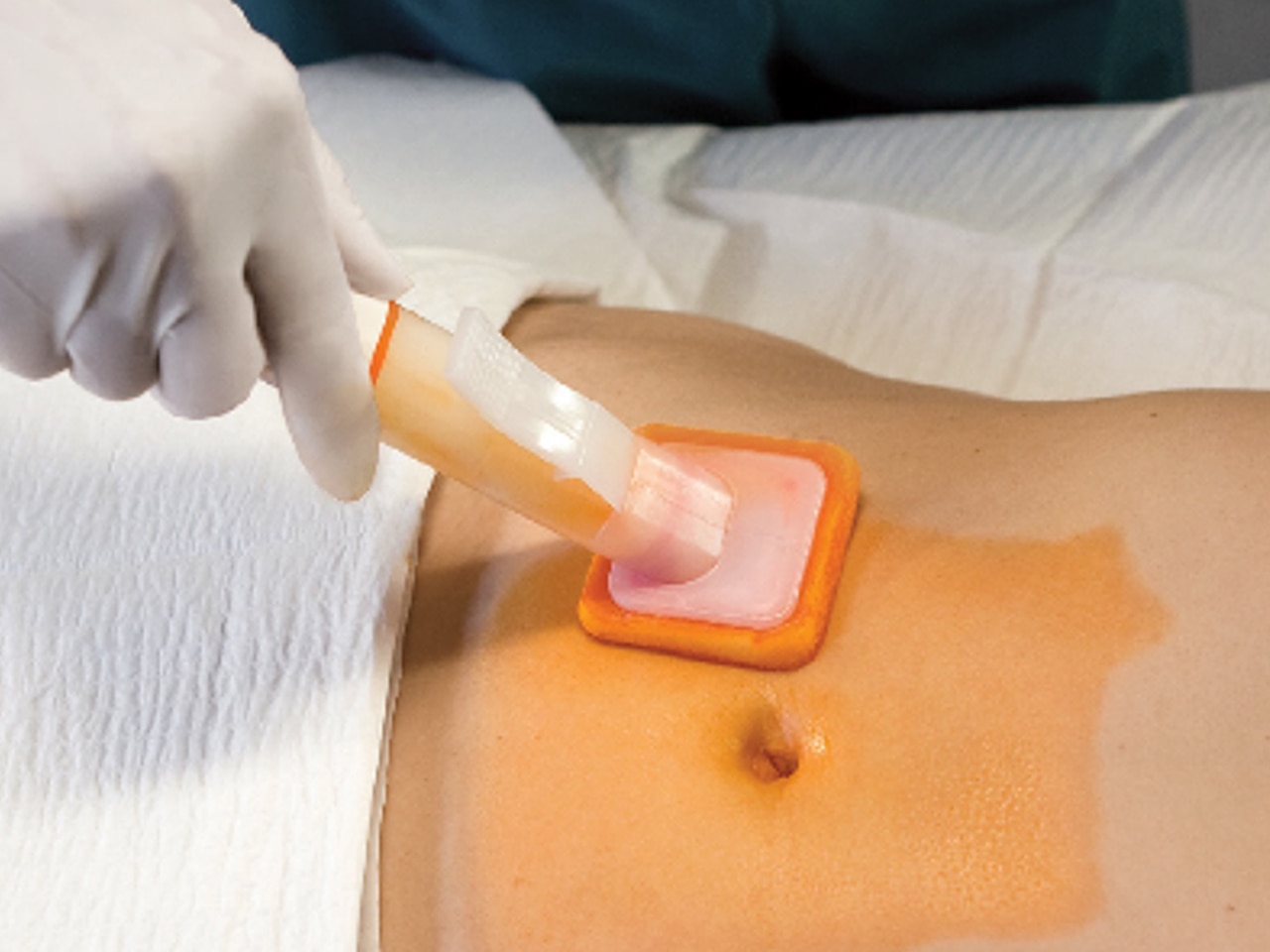
Hi-Lite Orange™ Tint

The Hi-Lite Orange™ advantage
In a surgeon/clinician survey (n=50), clinicians prefer BD ChloraPrep™ preoperative patient skin antiseptic with sterile solution and Hi-Lite Orange™ tint over other colored tint skin antiseptics because it helps mark the prep area and reduces the risk of clinical misdiagnosis based on skin coloring during post-op care. For example, a clinical misdiagnosis would be cyanosis, bruising, jaundice, and erythema.
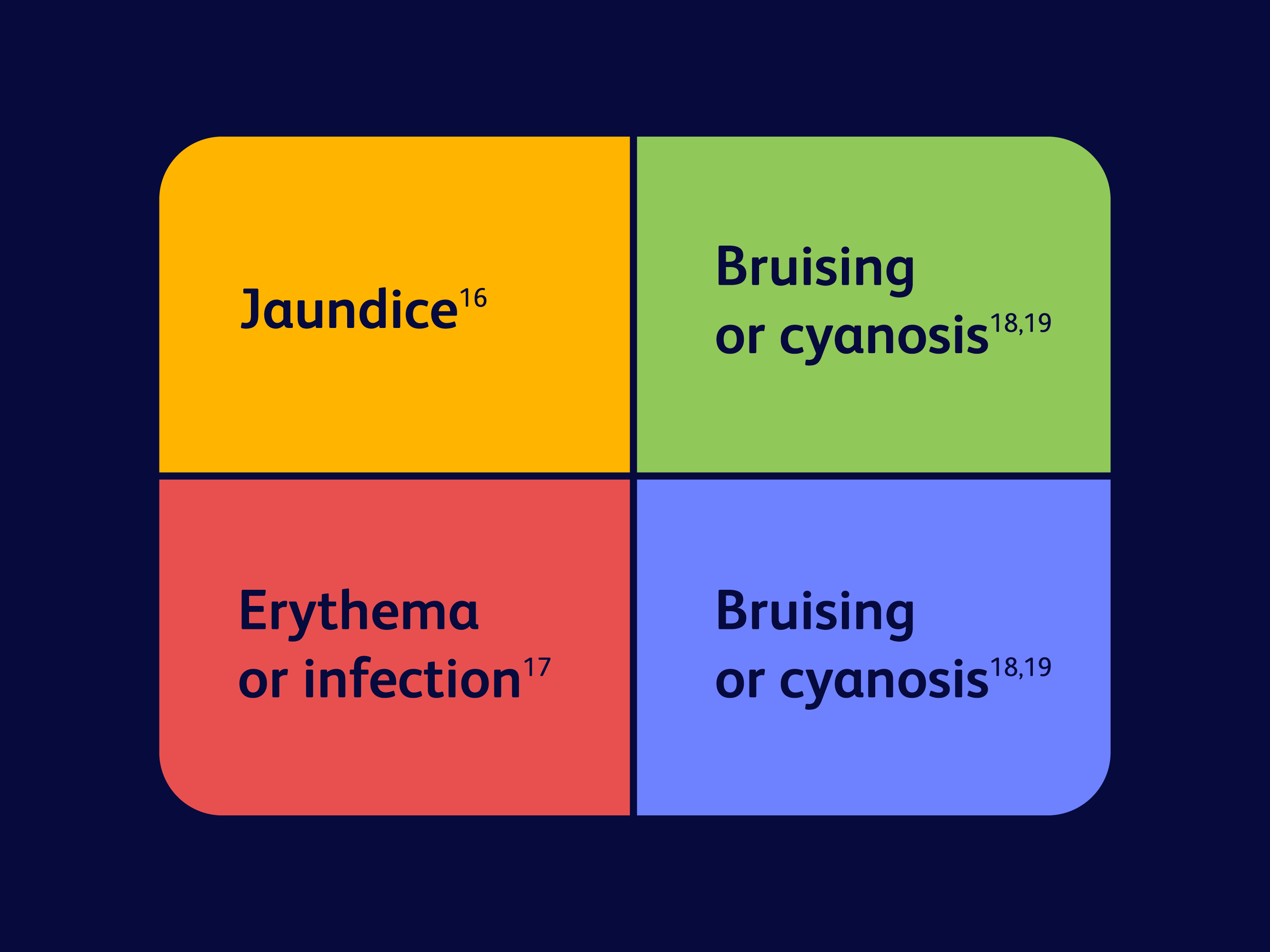
Why tint matters in post-op assessment
The impact of skin tint on clinical evaluation after surgery:
Certain tint colors—like red, green, yellow, or blue—may affect post-op assessment by potentially being misinterpreted as various conditions.
Guidance
The AORN Guidelines for Preoperative Patient Skin Antisepsis 4.4.5 recommends selecting the tinted skin antiseptic that is most visible on the individual patient’s skin.20
Please fill out and submit the form below, then we’ll be in touch.
AORN: Association of periOperative Registered Nurses; CHG: chlorhexidine gluconate; IPA: isopropyl alcohol; USP: United States Pharmacopeia.
References:
- Becton, Dickinson and Company. Data on file.
- Periop Today. Guidelines key takeaways for patient skin antisepsis. AORN. Published December 5, 2024. Accessed July 3, 2025. https://www.aorn.org/article/guidelines-key-takeaways-for-patient-skin-antisepsis.
- Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi:10.1128/AAC.00138-07.
- Lim CY, In J. Randomization in clinical studies. Korean J Anesthesiol. 2019;72(3):221-232. doi:10.4097/kja.19049.
- Jabbari S. The pinnacle of evidence: systematic reviews and their superior impact and reliability. Gastrointest Oncol Manag Care. 2024;1(1):2403790. doi:10.1080/29937817.2024.2403790.
- Pruka A. Evidence-based practice toolkit: levels of evidence. Winona State University, Krueger Library. Updated May 12, 2025. Accessed July 3, 2025. https://libguides.winona.edu/ebptoolkit/levels-evidence.
- Denton GW. Chlorhexidine. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:321-336.
- US Food and Drug Administration. Safety and effectiveness of health care antiseptics: topical antimicrobial drug products for over-the-counter human use (final rule). Published December 20, 2017. Accessed July 3, 2025. https://www.fda.gov/about-fda/economic-impact-analyses-fda-regulations/safety-and-effectiveness-health-care-antiseptics-topical-antimicrobial-drug-products-over-counter.
- desJardins-Park HE, Mascharak S, Chinta MS, Wan DC, Longaker MT. The spectrum of scarring in craniofacial wound repair. Front Physiol. 2019;10:322. doi:10.3389/fphys.2019.00322.
- Beausoleil C, Comstock SL, Werner D, Li L, Eby JM, Zook EC. Antimicrobial persistence of two alcoholic preoperative skin preparation solutions. J Hosp Infect. 2022;129:8-16. doi:10.1016/j.jhin.2022.08.008.
- Florman S, Nichols RL. Current approaches for the prevention of surgical site infections. Am J Infect Dis. 2007;3(1):51–61. doi:10.3844/ajidsp.2007.51.61.
- Elliott TS, Moss HA, Tebbs SE, et al. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur J Clin Microbiol Infect Dis.1997;16(3):210-213. doi:10.1007/BF01709583.
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223.
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674-686. doi:10.1111/j.1524-4725.2005.31612.
- US Food and Drug Administration. FDA drug safety communication. FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection. Published February 29, 2016. Accessed July 3, 2025. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requests-label-changes-and-single-use-packaging-some-over-counter.
- Mount Sinai. Jaundice. Mount Sinai Health System. Accessed July 3, 2025. https://www.mountsinai.org/health-library/diseases-conditions/jaundice.
- Nall R. What to know about skin redness. Healthline. Updated August 28, 2024. Accessed July 3, 2025. https://www.healthline.com/health/skin-redness.
- Fletcher J. What do the colors of a bruise mean? Medical News Today. Updated January 18, 2024. Accessed July 3, 2025. https://www.medicalnewstoday.com/articles/322742.
- Adeyinka A, Kondamudi NP. Cyanosis [archived]. StatPearls. Treasure Island, FL: StatPearls Publishing; August 12, 2023.
- AORN. Guideline for preoperative patient skin antisepsis. In: Guidelines for Perioperative Practice. Denver, CO: AORN, Inc; 2021.