There’s no room for compromise in the OR
When the life patients want to go back to living is at stake, second best won’t cut it. With a portfolio of reliable and effective surgical products, BD gives surgeons and their patients one less thing to worry about. Explore our products and solutions today.

Hear Our Perspective
Discover products made without compromise

BD® ChloraPrep™ skin preparation
A patient preoperative skin preparation that delivers standardized, powerful, persistent antimicrobial protection that is backed by more than 60 clinical studies and trusted by healthcare providers for more than 25 years.
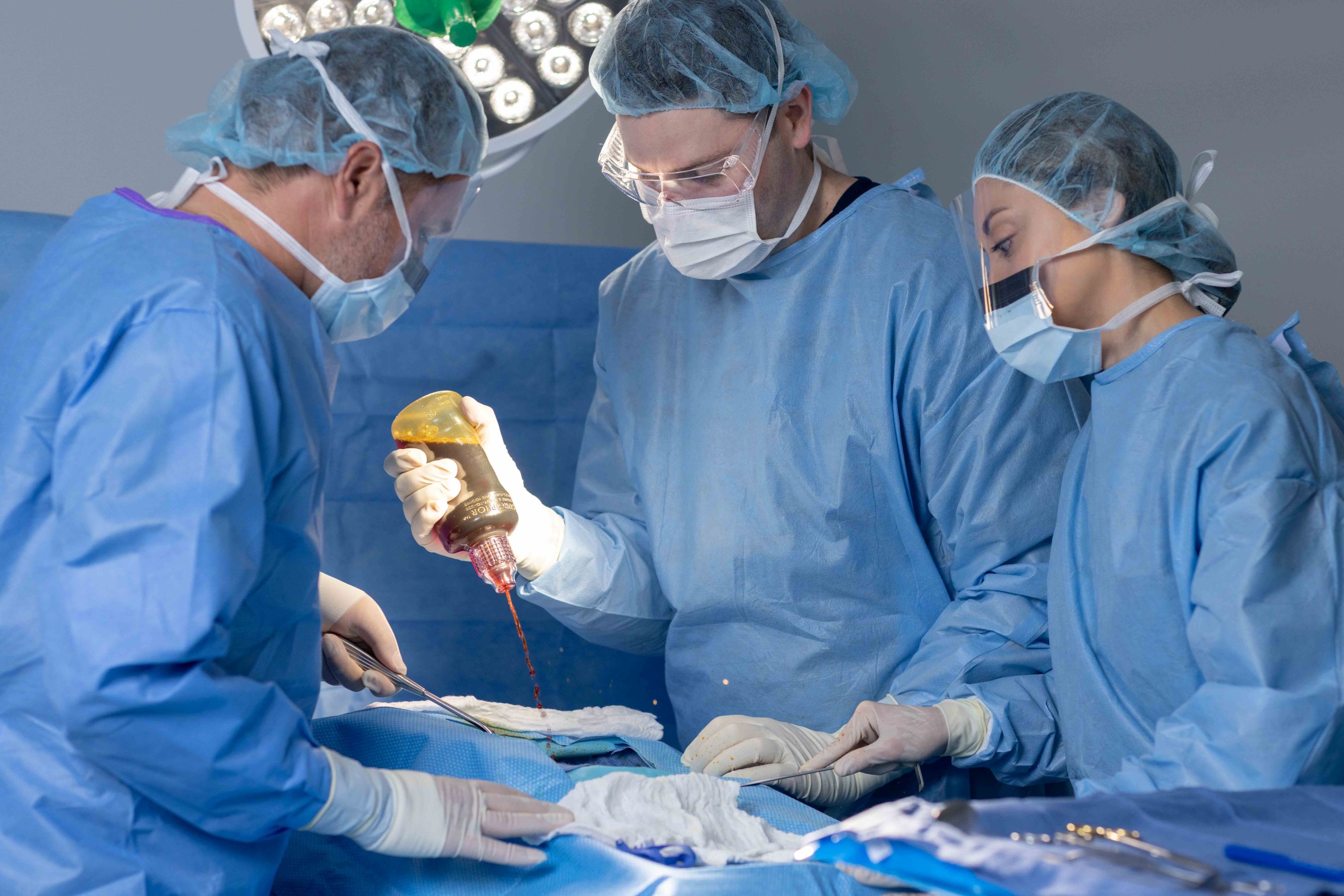
BD® Surgiphor™ Antimicrobial irrigation system
A ready-to-use, terminally sterile, surgical irrigation solution that delivers a dilute povidone-iodine (PVP-I) lavage. Surgiphor™ Irrigation System is designed to reduce variability in process, maintaining consistent concentration and supporting best practices in surgical irrigation.
BD® Arista™ AH
A 100% plant-based absorbable surgical hemostatic powder derived from purified and hydrolyzed plant starch. The power of Arista™ AH lies in its Microporous Polysaccharide Hemospheres, a patented blood clotting technology.

Explore the BD® Power in Prevention program
The BD Power in Prevention program can help standardize pre-op practices. Download this overview of the program to see how it could improve your outcomes.
Get in touch to discuss BD surgical products today
BD and the BD Logo are trademarks of Becton, Dickinson and Company or its affiliates. © 2025 BD. All rights reserved. BD-154346 (08/25)
Arista™ AH Absorbable Hemostat
INDICATIONS
Arista™ AH is indicated in surgical procedures (except neurological and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
CONTRAINDICATIONS
Do not inject or place Arista™ AH into blood vessels as potential for embolization and death may exist.
WARNINGS
Arista™ AH is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
Once hemostasis is achieved, excess Arista™ AH should be removed from the site of application by irrigation and aspiration particularly when used in and around foramina of bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm.
Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. Dry, white Arista™ AH should be removed. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.
Safety and effectiveness of Arista™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
Arista™ AH should be used with caution in the presence of infection or in contaminated areas of the body. If signs of infection or abscess develop where Arista™ AH has been applied, re-operation may be necessary in order to allow drainage.
Safety and effectiveness in neurosurgical and ophthalmic procedures has not been established.
Arista™ AH should not be used for controlling post-partum bleeding or menorrhagia.
PRECAUTIONS
When Arista™ AH is used in conjunction with autologous blood salvage circuits, carefully follow instructions in the Administration section of the IFU regarding proper filtration and cell washing.
Arista™ AH is intended to be used in a dry state. Contact with saline or antibiotic solutions prior to achieving hemostasis will result in loss of hemostatic potential.
Arista™ AH is not recommended for the primary treatment of coagulation disorders.
No testing has been performed on the use of Arista™ AH on bone surfaces to which prosthetic materials are to be attached with adhesives and is therefore not recommended.
Arista™ AH is supplied as a sterile product and cannot be resterilized. Unused, open containers of Arista™ AH should be discarded.
Do not apply more than 50g of Arista™ AH in diabetic patients as it has been calculated that amounts in excess of 50g could affect the glucose load.
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
ADVERSE REACTIONS
None of the adverse events that occurred in a randomized prospective, concurrently controlled clinical trial were judged by the Data Safety Monitoring Board to be related to the use of Arista™ AH. The most common recorded adverse events were pain related to surgery, anemia, nausea, lab values out of normal range, arrhythmia, constipation, respiratory dysfunction and hypotension – all reported in greater than 10% of the Arista™ AH treated patients. The details of this clinical trial’s adverse events can be reviewed in the IFU supplied with the product and are also available at www.bd.com.
Caution: Federal (USA) law restricts this device to sale by or on order of a licensed physician or properly licensed practitioner.
BD® ChloraPrep™ Patient Preoperative Skin Preparation
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use. © 2025 BD. BD, the BD logo, the Sterile Solution logo, ChloraPrep and PurPrep are trademarks of Becton, Dickinson and Company. All other trademarks are the property of their respective owners. This document and the information contained herein is for general information purposes only and is not intended, and does not constitute legal, reimbursement, business, or other advice. Furthermore, it does not constitute a representation or guarantee of cost-effectiveness, and it is not intended to increase or maximize payment by any payer. Nothing in this document should be construed as a guarantee by BD regarding cost-effectiveness, expenditure reduction, reimbursement or payment amounts, or that reimbursement or other payment will be received. The ultimate responsibility for determining cost-effectiveness and obtaining payment/reimbursement remains with the customer. This includes the responsibility for accuracy and veracity of all claims submitted to third-party payers. Also note that actual costs for products and services and any related expenditures vary, and that the information presented herein represents only one of many potential scenarios, based on the assumptions, variables, and data presented. In addition, the customer should note that laws, regulations, and coverage policies are complex and are updated frequently, and, therefore, the customer should check with its local carriers or intermediaries often and should consult with legal counsel or a financial or reimbursement specialist for any questions related to cost-effectiveness, expenditure reduction, billing, reimbursement or any related issue.
BD® Surgiphor™ Antimicrobial Irrigation System
DESCRIPTION: An antimicrobial irrigation system containing 0.5% povidone (PVP-I) in phosphate-buffered saline, potassium iodide and Vitamin E TPGS. PVP-I acts as a preservative to help inhibit microbial growth in the irrigation solution.
INDICATION FOR USE: Surgiphor™ Antimicrobial Irrigation System is intended to mechanically loosen and remove debris, and foreign materials, including microorganisms, from wounds.
CONTRAINDICATIONS: Surgiphor™ Antimicrobial Irrigation System should not be used in patients with known allergic reaction to any of the ingredients in the solutions. Surgiphor™ Antimicrobial Irrigation System should also not be combined with other irrigation or antiseptic solutions due to potential reactions and reduction in the effectiveness of the system. Not for use in neonates.
WARNINGS: Do not use or mix with other cleansers, soaps, lotions, or ointments. Do not use for injection or infusion. Do not swallow. Do not use in eyes or ear canals. Discontinue use immediately if irritation or an allergic reaction occurs. Do not use if packaging is damaged or if seal integrity is compromised. Do not reuse Surgiphor™ solution after 24 hours.
PRECAUTIONS: Surgiphor™ solution may cause a temporary irritation and/or burning sensation on exposed skin in very rare cases. Surgiphor™ solution may cause allergic reactions such as rash or skin irritation in patients with iodine allergy. Anaphylaxis with the use of Surgiphor™ solution may occur in patients with severe iodine allergy. Federal law restricts this device to sale by or on the order of a licensed physician. Single patient use only. Not for at-home use. Please consult product insert for complete indications, contraindications, warnings, precautions, safety information and instructions for use.