Contact Support
Clinically proven to perform under pressure1
Tridyne™ Cardiovascular Sealant is the latest addition to the BD family of hemostasis solutions, offering cardiovascular, cardiothoracic, and vascular surgeons a unique, easy-to-use solution to reinforce aortic anastomoses and control bleeding when adjunctive measures are required. By combining a proprietary formulation of polyethelyne glycol and recombinant human albumin (rHA), it forms a strong, flexible seal, even in anticoagulated patients.1,2 The Tridyne™ hydrogel is designed to adhere where it should – on both tissue and grafts.*
Preclinical data demonstrates that Tridyne™ Cardiovascular Sealant resorbs naturally to maintain its seal strength through the critical postoperative period without compromising tissue healing.2
Tridyne™ Cardiovascular Sealant provides application control without the need for pressurized gas.
The Tridyne™ applicator is designed to:
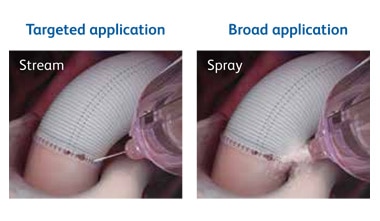
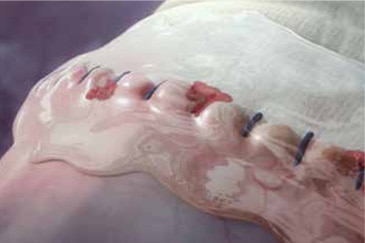
The specialized Tridyne™ formulation is transparent and forms a completely clear hydrogel at the application site. With its unique combination of clarity and coverage, Tridyne™ Cardiovascular Sealant allows for visual confirmation of hemostasis at the anastomotic suture line—making it an ideal choice for aortic surgery.
In a prospective, randomized, controlled multicenter study, Tridyne™ Cardiovascular Sealant outperformed Gelfoam® Plus Hemostasis Kit in several critical measures.6 The study included 156 patients from 18 different centers who underwent thoracic aortic surgery, with hemostasis evaluated at the aortic anastomotic suture line.1
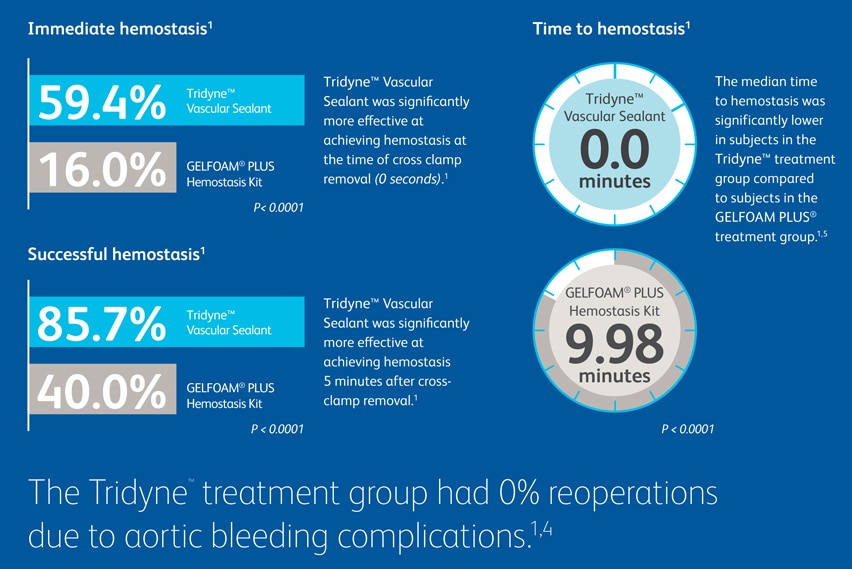
Tridyne™ Cardiovascular Sealant offers surgeons a specialized, easy-to-use solution during aortic repair, with unique application control and clinically proven results.1 With its demonstrated ability to provide fast, effective control of bleeding, it’s the sealant of choice for aortic surgery.
| SKU/REF | SKU/REF Name | Packaging |
|---|---|---|
| TDCV004 | Tridyne™ Cardiovascular Sealant (4 mL) | 4/cs |
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
* The use of Tridyne™ Vascular Sealant with ePTFE grafts or vascular patches has not been studied clinically. Preclinical data on file. Preclinical test results may not correlate to clinical performance.
INTENDED USE / INDICATIONS FOR USE
Tridyne™ Cardiovascular Sealant is indicated for use as an adjunct to hemostasis for treatment of cardiovascular and peripheral vasculature diffuse persistent bleeding by mechanically sealing areas of leakage.
CONTRAINDICATIONS
WARNINGS
ADVERSE EVENTS
The following potential Adverse Events have been associated with this type of surgical sealant:
In addition, the following potential Adverse Events and Serious Adverse Events have been associated with cardiac and vascular procedures.
BD-54058