Contact Support
A clinically proven umbilical hernia repair solution designed for ventral, incisional, umbilical and epigastric hernia repair as well as trocar site closure, with an absorbable barrier featuring Sepra® technology.
A clinically proven umbilical hernia repair solution with SorbaFlex™ Memory Technology and an absorbable barrier featuring Sepra® Technology.
Uncoated Mesh
Monofilament polypropylene mesh designed to allow a prompt fibroblastic response through the open interstices of the mesh.

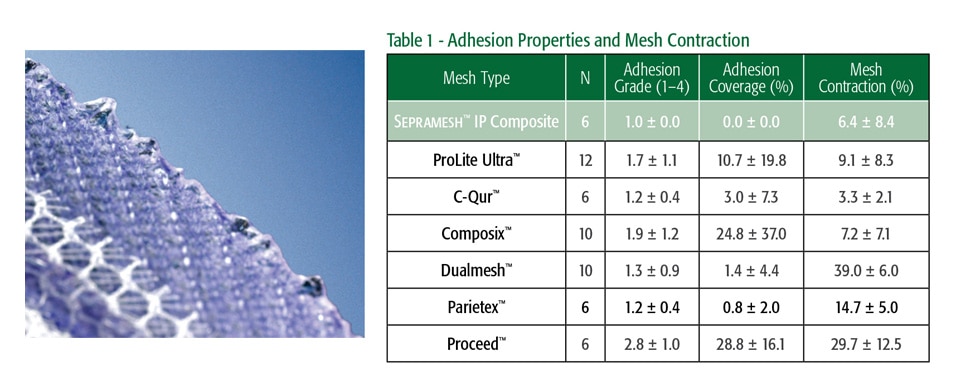
Sepramesh™ IP Composite
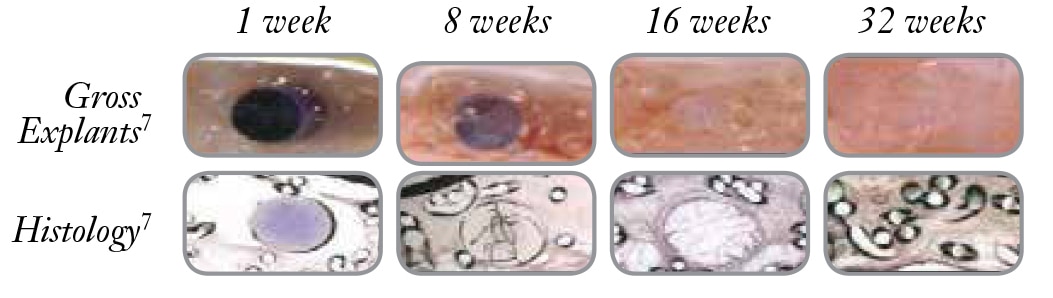
Unique hydrogel barrier, based on the Sepra® Technology, swells to minimize tissue attachment to the visceral side of the mesh and resorbs within 30 days providing visceral protection during the critical healing process.3
Sepramesh™ IP Composite Preclinical Study2,3
“120-Day Comparative Analysis of Adhesion Grade and Quantity, Mesh Contraction, and Tissue Response to a Novel Omega-3 Fatty Acid Bioresorbable Barrier Macroporous Mesh After Intraperitoneal Placement.”
SorbaFlex™ Memory Technology
Polydioxanone (PDO) monofilament is unique in its flexibility and tensile strength, facilitating patch insertion and placement. Preclinical testing has demonstrated that absorption via hydrolysis is essentially complete in 6-8 months.4
Unique Pocket and Strap Design
Pocket and strap facilitate placement, positioning and lateral fixation. SorbaFlex™ Memory Technology is contained within a knitted polypropylene mesh tube.
Easy
Minimum dissection, fixation required.
Efficient
Proprietary pocket and strap design facilitates placement, positioning and lateral fixation.
Proven
Clinically supported technique since 2002 with over 800,000 implants worldwide and peer-reviewed published clinical studies.

Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Indications.
Ventralex™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving the repair of ventral, incisional, and umbilical hernias.
Contraindications.
Warnings.
Precautions.
Adverse Reactions.
Possible complications may include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection. Please consult package insert for more detailed safety information and instructions for use.