Contact Support
Choose the only needle-free connector with an FDA-cleared label statement demonstrating a reduction in CLABSI
Hospitals using the BD MaxPlus™ needle-free connector had lower unadjusted central line-associated bloodstream infection (CLABSI) rates, as well as lower standardised infection ratios, compared to hospitals not using the MaxPlus technology.*1

The solid sealed surface withstands 7 days/200 activations.

The clear design shows the complete fluid pathway, enabling optimal visibility when flushing.

Zero interstitial space between the components limits opportunities for microbial ingress.
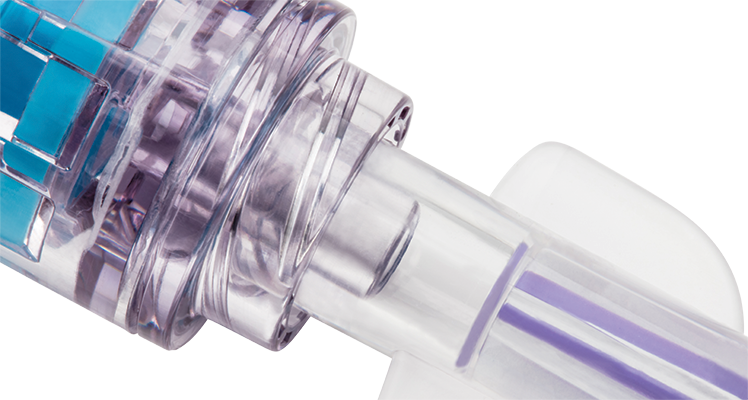
Zero-reflux feature upon disconnection can help reduce catheter occlusions and catheter occlusions
SKU/REF |
Description |
Priming Volume (mL) |
Pressure Rating |
Change Interval |
Quantity |
Unit measure |
| MP1000- 0006 |
BD MaxPlus™ needle-free connector |
0.28 | 325 PSI @ 10 mL per second |
7 days/200 activations |
100 | Per case |
| MP1000C- 0006 |
BD MaxPlus™ clear needle-free connector | 0.28 | 325 PSI @ 10 mL per second |
7 days/200 activations |
100 | Per case |
Visit online catalogue
In our online catalogue, you can easily view our offering of:
View full IV Disposable portfolio »
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
![]()
* 2013 CMS hospital compare data from 3,074 hospitals, accounting for nearly 11,000 CLABSIs associated with nearly 10 million catheter days.
BD-35577 (08/2021)