Contact Support
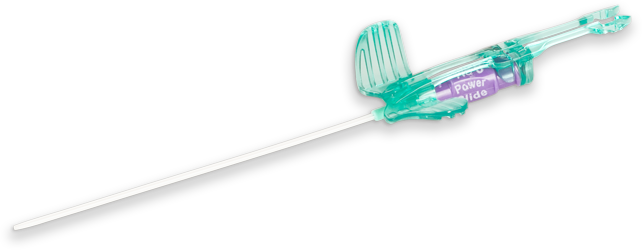
As a fully integrated placement device, the PowerGlide Pro™ Midline Catheter is designed to be a simplified solution for peripheral IV therapy.

Reinforced Tip Catheter
Designed to improve and maintain a consistent aspiration flow rate without catheter collapse.2

Body-softening polyurethane catheter
The body of the polyurethane catheter softens at body temperature, helping to minimize vessel wall trauma.
Integrated Guidewire
The integrated nitinol guidewire is designed to aid with difficult sticks and allows for steep angles of insertion.
Each hub is marked with the PowerGlide™ name and the catheter length, helping to eliminate confusion.
Power injectableThe catheter allows for the injection of contrast media for CT scans at maximum flow rates:

Catheter wings contain a blood control mechanism that impedes blood flow until the clinician is ready to attach an accessory device.
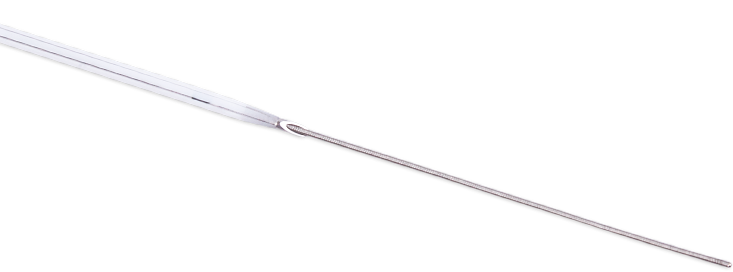
A notched needle allows visual confirmation of flashback before it reaches the flash chamber.
| Item Description | Basic Tray | Full Tray |
|---|---|---|
| PowerGlide Pro™ Midline Catheter | • | • |
| StatLock™ Stabilization Device | • | • |
| Skin Prep Pad | • | • |
| Transparent Dressing | • | |
| Absorbent Towel | • | |
| Measuring Tape | • | |
| Mask | • | |
| Gauze | • | |
| Tourniquet | • | |
| Gloves | • | |
| Absorbent Drape | • | |
| Fenestrated Drape | • | |
| Extension Set | • |
In our online catalogue, you can easily view our offering of:
View full Midline Catheter portfolio »
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
![]()
Please consult product labels, IFU, and package inserts for any indications, contraindications, hazards, warnings, cautions, and instructions for use.
BD-60985