Same proven technique with improved mesh.
OnFlex™ Mesh was specifically designed to fit the inguinal anatomy during preperitoneal placement. It offers extended medial and inferior coverage for direct and femoral hernia spaces.
Self-expanding lightweight mesh for open preperitoneal inguinal hernia repair with SorbaFlex™ Memory Technology
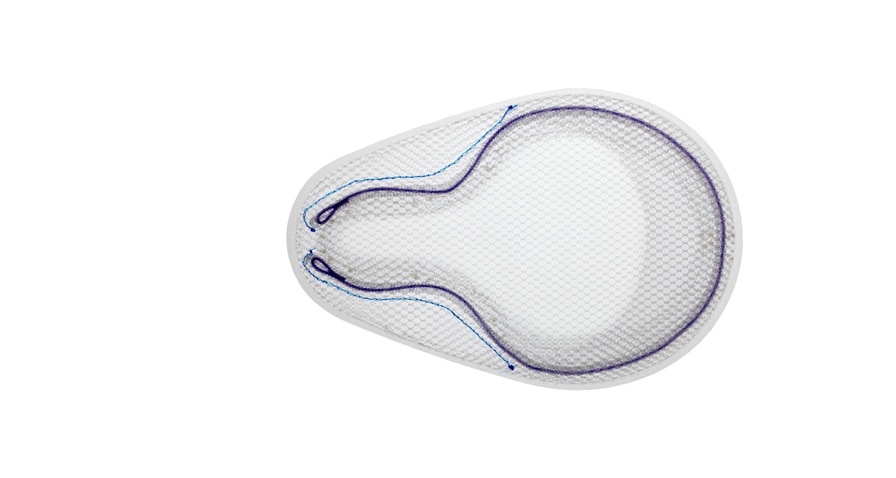

OnFlex™ Mesh was specifically designed to fit the inguinal anatomy during preperitoneal placement. It offers extended medial and inferior coverage for direct and femoral hernia spaces.

Lightweight, Large Pore Mesh
Positioning Pocket
Absorbable SorbaFlex™ Memory Technology
Interrupted PDO Monofilament
Inguinal Notch
Brown C, Finch J. Which mesh for hernia repair? Annals of The Royal College of Surgeons of England 2010;92(4):272-278.
Disclaimers
Indications
The OnFlex™ Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, such as in the repair of inguinal hernias.
Contraindications
Do not use this mesh in infants, children, or pregnant women, whereby future growth may be compromised by the use of such mesh material. The use of this mesh has not been studied in breastfeeding or pregnant women. Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, allergic reaction, and recurrence of the hernia or soft tissue defect. The mesh must be removed immediately if the SorbaFlex™ PDO monofilament is cut or damaged during insertion of fixation. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include, but are not limited to, bowel or skin perforation and infection.
Warnings
BD-113490 (03/2024)