Seal with confidence
TissuePatchDural™ is a self-adhesive film indicated for use to seal and reinforce against fluid (including CSF) and/or blood leakage, where repair of the dura mater is required.1
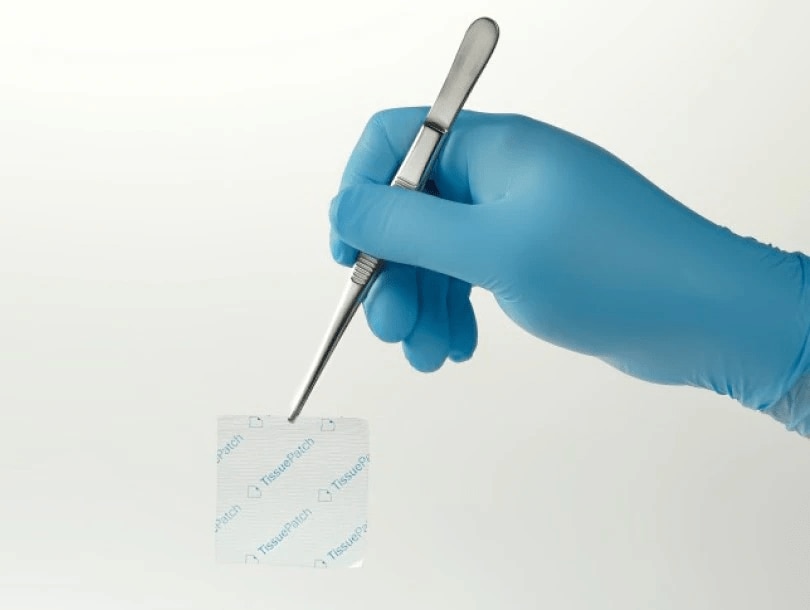

TissuePatchDural™ is a self-adhesive film indicated for use to seal and reinforce against fluid (including CSF) and/or blood leakage, where repair of the dura mater is required.1
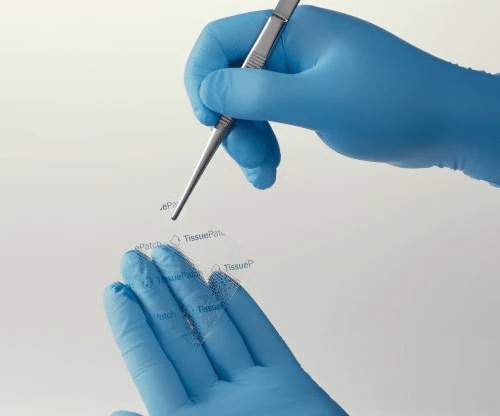
SELECT with confidence
**Shown in pre-clinical models.
PLACE with confidence
TissuePatchDural™ is only 0.04 mm thick and transparent1

SEAL with confidence
TissuePatchDural™ is a self-adhesive patch
*Compared to DuraSeal™ and Tachosil™ Fibrin Sealant Patch.
TissuePatchDural™ Sealant Film Application
TissuePatchDural™ following redo craniotomy video
TissuePatchDural™ In vitro Handling video
TissuePatchDural™ used for closure of midline spinal durotomy video
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Indications for use: TissuePatchDural™ is indicated for use to seal and reinforce against fluid (including CSF) and/or blood leakage where the repair of the dura mater is required. TissuePatchDural™ is intended for use as an adjunct.
Contra-indications for use: TissuePatchDural™ is not intended for intravascular use; not intended to be used as a dura mater substitute; not intended to replace sutures, staples or clips, as appropriate, in tissue approximation.
Warnings and safety information:
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings, and precautions.
BD-83468