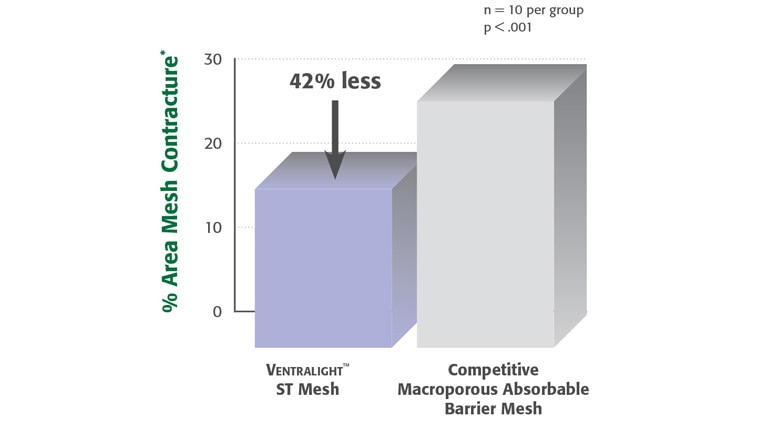
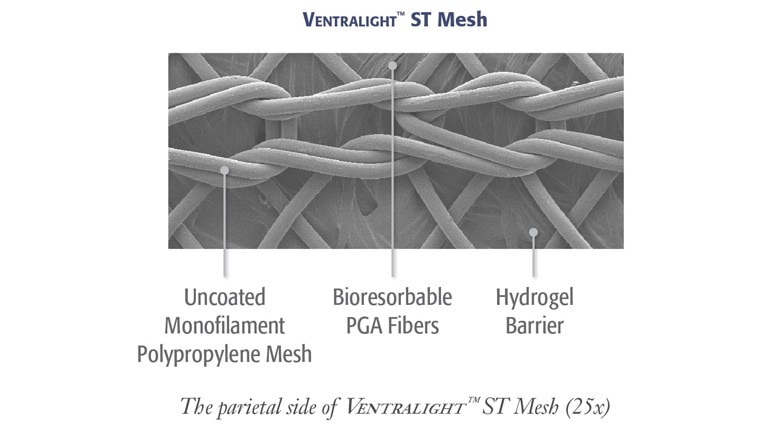
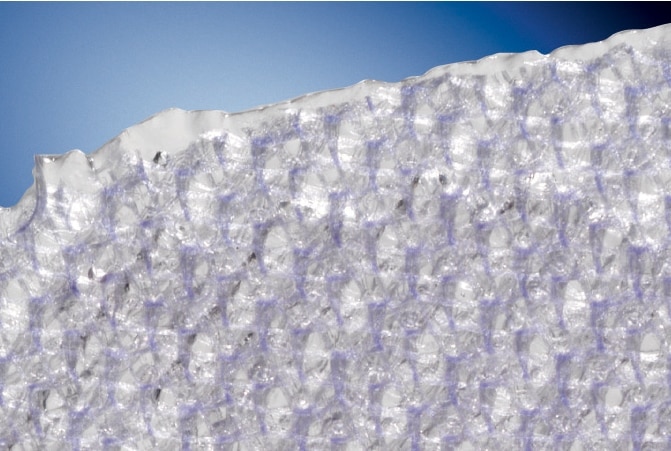
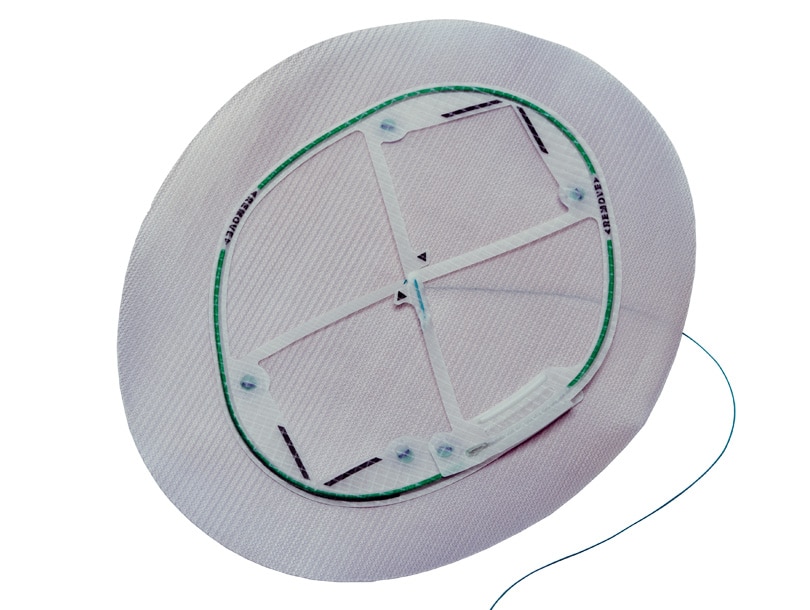
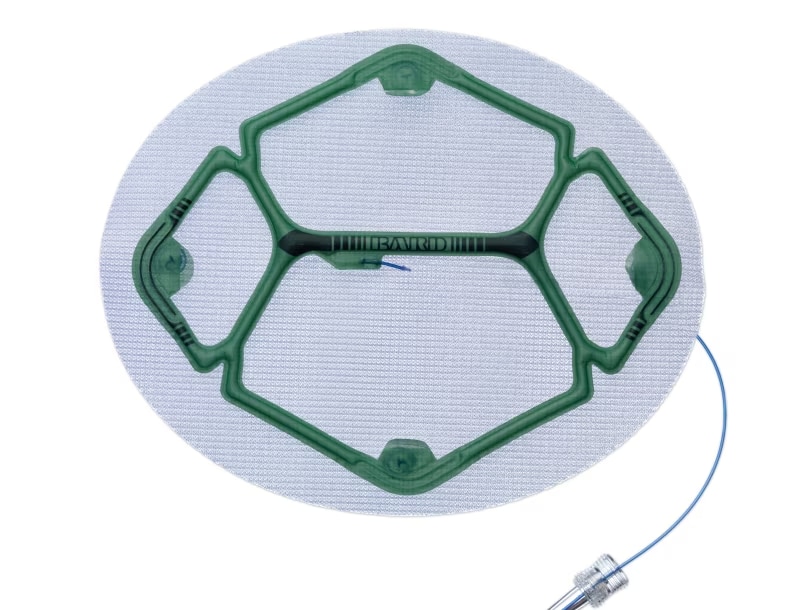
Proven Sepra® Technology in a Low Profile, Medium Weight Mesh
Ventralight™ ST Mesh is an uncoated medium weight monofilament polypropylene mesh on the anterior side with an absorbable hydrogel barrier based on Sepra® Technology on the posterior side for laparoscopic ventral hernia repair.