Designed to enable constructive and functional tissue remodeling1
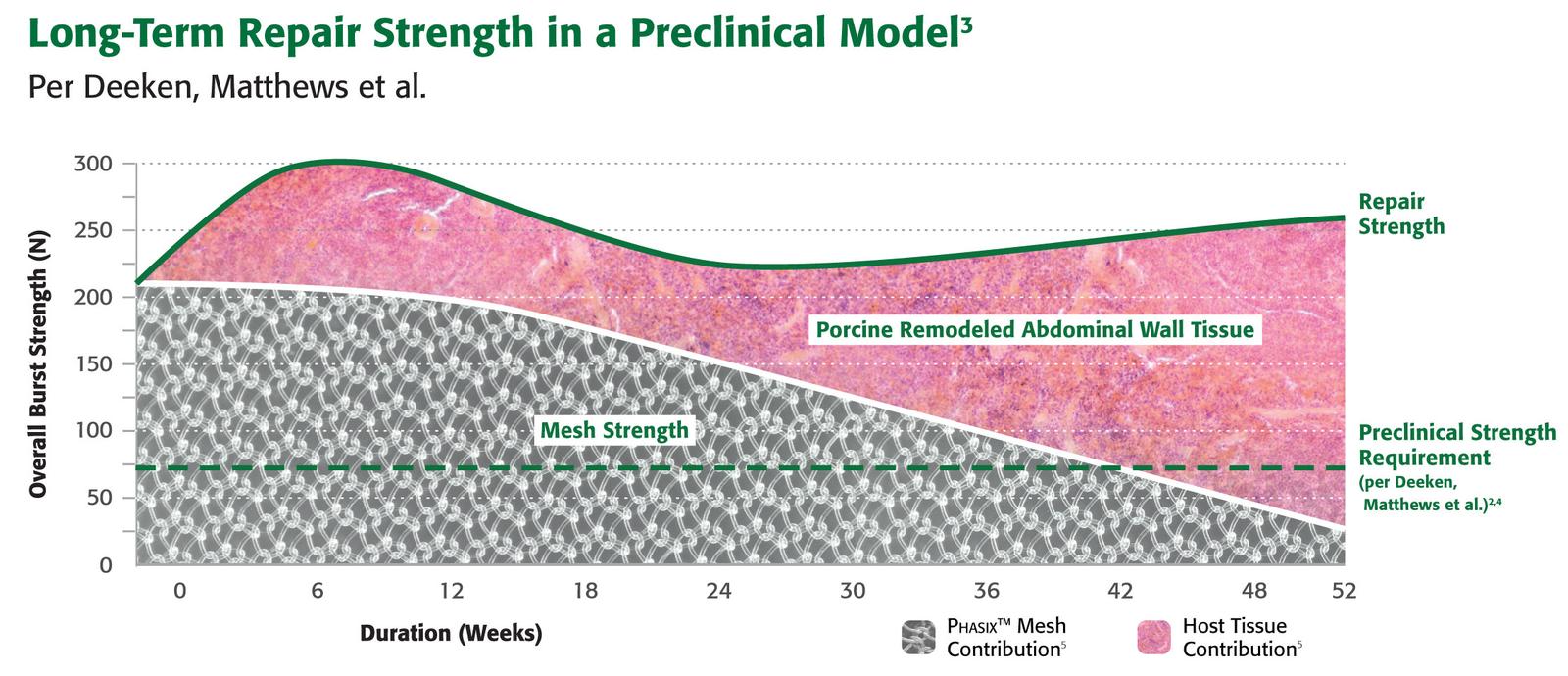
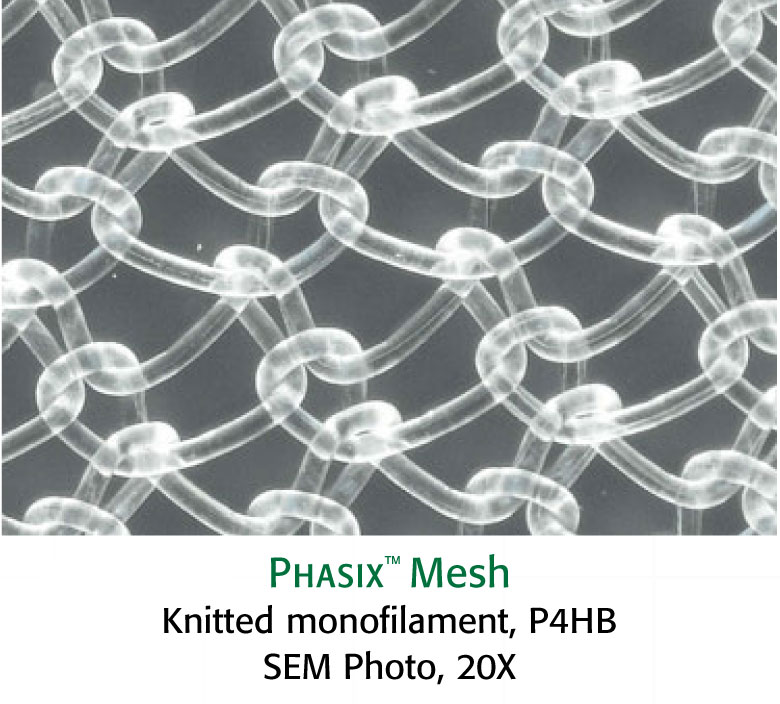
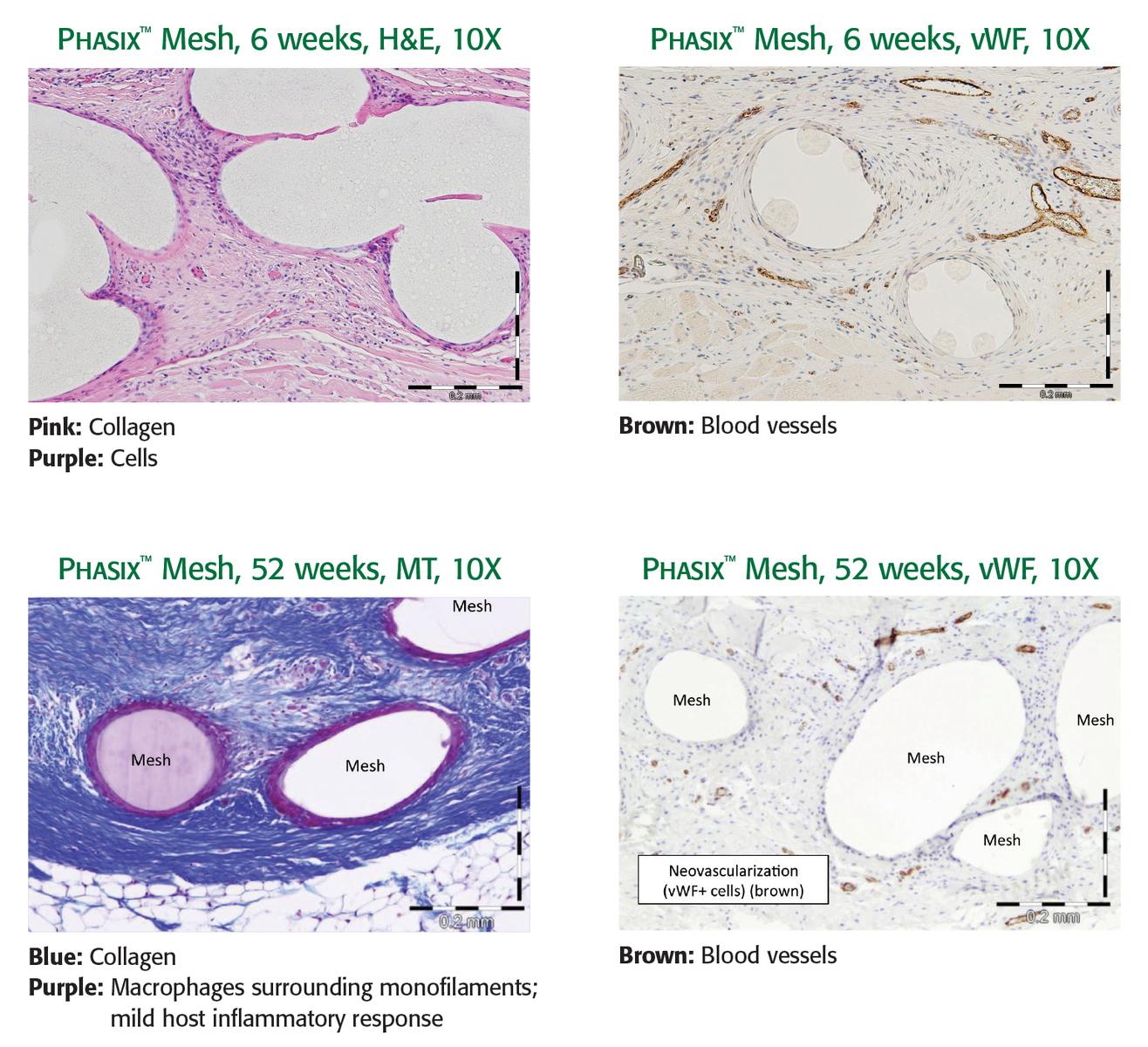
Phasix™ Mesh provides a fully resorbable monofilament scaffold for rapid tissue incorporation that has been designed to allow for the repair strength of a synthetic mesh along with the remodeling characteristics of a biologic graft.1