Phasix™ ST Mesh
A fully Bioresorbable Poly-4-hydroxybutyrate (P4HB) scaffold featuring proven Sepra® technology barrier to adhesions.


- Overview
- EIFU & Resources
Designed to enable functional tissue remodeling for a strong repair
Phasix™ ST Mesh combines two market-leading technologies into one product: monofilament resorbable Phasix™ Mesh and a proven hydrogel barrier based on Sepra® technology. While the monofilament mesh supports functional healing and a strong repair, the hydrogel barrier minimises tissue attachment to the visceral side of the mesh for intraabdominal placement.1
The next phase in hernia repair
Repairs.1
The open monofilament mesh structure provides early integration and repair strength.1
Remodels.1
Vascular integration and incorporation continues, with abundant mature collagen at 52 weeks. Gradually transfers load to native tissue over time. 1
Restores.1
As Phasix™ Mesh is remodeled, it is replaced with functional tissue, ultimately resulting in a strong repair at one year.1
Material structure1
Monofilament mesh designs have been deemed more biocompatible and less susceptible to bacterial adherence and colonization.1
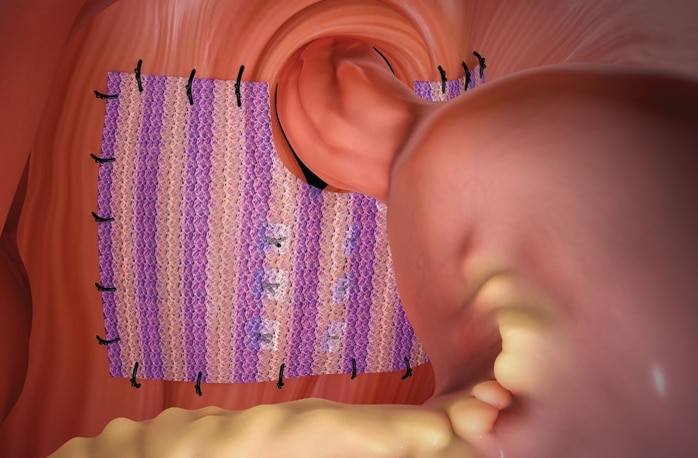
Phasix™ ST Mesh offers a durable repair without permanent material. The only bioresorbable mesh with a proven hydrogel barrier for hiatal hernia repair.1 Phasix™ ST Mesh prospective study on paraesophageal hernia repair with 12-month follow-up2
- 50 Patients
- Low recurrence rate of 8% with no mesh related complications or erosions
References
Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Disclaimers
- Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
- Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of abdominal soft tissue, where weakness exists, in ventral and hiatal hernia repair procedures.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Adverse Reactions
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, haematoma, pain, infection, inflammation, allergic reaction, haemorrhage, extrusion, erosion, migration, fistula formation and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include oesophageal erosion and dysphagia related to crural fibrosis.
Warnings
- Mesh manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this mesh in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
- Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
- The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established.
- The safety and effectiveness of Phasix™ ST Mesh in laparoscopic/robotic ventral hernia repair procedures has not been evaluated or established.
- The use of any mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
BD-113492 (06/23)