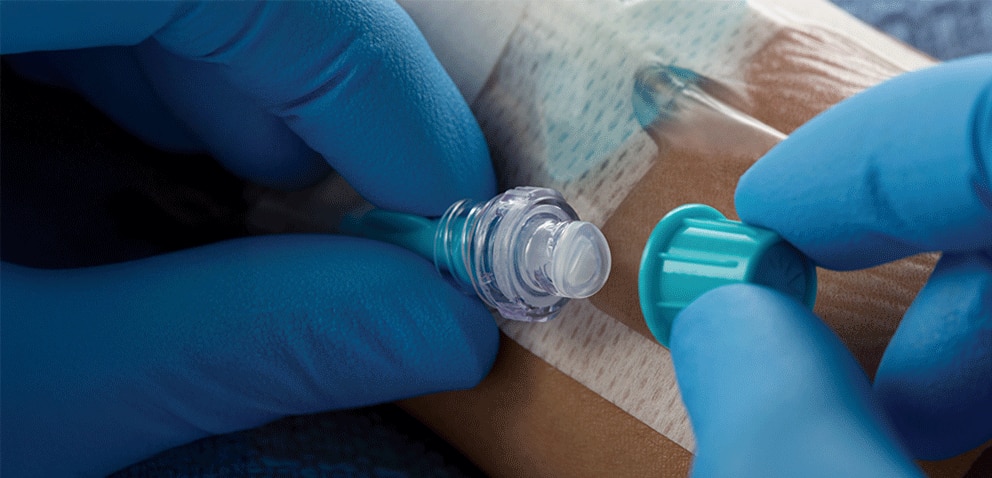
Q-Syte™ Needle-free Connectors and Extension Sets are intended for use with vascular access devices for intravascular administration of fluids.1

- Overview
- Products & Accessories
- eIFU & Resources
-
Diverse applications
The split-septum design of the Q-Syte™ Needle-free Connector is compatible with both luer slip and luer lock devices.
-
High flow rates
The Q-Syte™ Needle-free Connector helps enable high-volume infusions with a flow rate of 32 L/hr.
-

Easy to use
100% of clinicians considered the Q-Syte™ Needle-free Connector easy to use in a simulated use study (n=11) compared to PRN adaptors*.
According to CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, a split-septum needle-free connector design may be preferred over mechanical valves.¹ The Q-Syte™ Needle-free Connector features a split-septum design and has been shown to help reduce the risk of central line-associated bloodstream infections (CLABSI) when used as part of a catheter-related bloodstream infection (CRBSI) prevention bundle.2,3

Available in single-fuse and multi-fuse options with a broad range of configurations.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
* Data on file: Galahad Device Marketing Simulation Study TS02870, 2000. Clinician Response to Questionnaire Summary.
- Q-Syte™ Luer Access Split Septum. Technical Data Sheet, 2016.
- Rosenthal VD, Udwadia FE, Kumar S, et al. Clinical impact and cost effectiveness of split-septum and single-use prefilled flushing device vs 3-way stopcock on central-line associated bloodstream infection rates in India: a randomized clinical trial conducted by the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control. 2015;43(10):1040–1045. doi: 10.1016/j.ajic.2015.05.042
- Devrim I, Özkul MT, Çağlar I et al. Central line bundle including split-septum device and single-use prefilled flushing syringes to prevent post-associated infections: a cost and resource- utilization analysis [published correct appears in BMC Health Serv Res. 2020:20(1):336]. Published correction in BMC Health Serv Res. 2020:20(1):450. doi:10.1186/s12913-030-05221-6.
