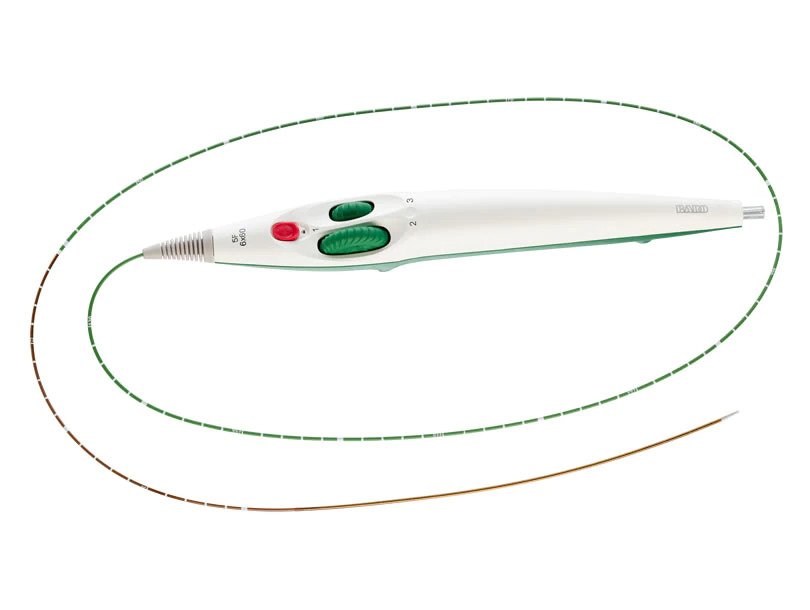
Helical design
Indicated for SFA and popliteal artery
Low profile, 5F delivery system
Dual-speed thumbwheel deployment designed for ease of use and placement accuracy2





- Overview
- Products & Accessories
- EIFU & Resources
LifeStent™ Vascular Stent has a history of proven performance:
- In a Level 1 RESILIENT study, the LifeStent™ Vascular Stent demonstrated treatment superiority over balloon angioplasty with sustained effectiveness out to 3 years4
- In the investigator initiated, Level 1 ETAP study of the popliteal artery the LifeStent™ Vascular Stent demonstrated double the primary patency rate of PTA out to two years5
- In a clinical assessment of the treatment of long lesions, the LifeStent™ Vascular Stent demonstrated high primary patency at 12 months in lesions up to 240mm6
The LifeStent™ Vascular Stent Systems, in varying sizes, have been studied in more than ten clinical trials globally.7
The LifeStent™ 5F Vascular Stent System offers the same clinically-proven advanced helical stent design as the LifeStent™ Vascular Stent on a low profile, 5F delivery system. The LifeStent™ 5F delivery system offers dual speed thumbwheel deployment with a tri-axial catheter that is designed for ease of use, deployment control, and precise placement accuracy.2
Peripheral stent intended to improve luminal diameter
The LifeStent™ 5F Vascular Stent System is a peripheral stent intended to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions in the native superficial femoral artery (SFA) and popliteal artery. The LifeStent™ 5F Vascular Stent System is available in 5 mm, 6 mm, and 7 mm diameters; and 20 mm to 170 mm in length. Refer to the specifications table for specific size options.
1 Commercially available as of December 2023
2 Based on physician ratings during animal testing. May not be indicative of clinical performance. Data on file at Bard Peripheral Vascular, Inc., Tempe, AZ.
3 The GeoAlign™ Marking System provides an approximation that may not be an exact representation of the distance traveled intravascularly and should be confirmed under fluoroscopy.
4 Freedom from TLR at 3 years: 75.5% LifeStent™ Vascular Stent arm (n=134), 41.8% PTA arm (n=72), p LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 40-80 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
5 Primary Patency at 2 years: 64.2% LifeStent™ Vascular Stent arm (n=89), 31.3% PTA arm (n=94), p=0.0001. Patency rates calculated when provisional stenting is considered TLR. Kaplan-Meier analysis with Mantel-Cox log-rank test. The study included LifeStent™ Vascular Stent in 6 mm, 7 mm and 8 mm diameters and lengths of 20-170 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
6 Primary Patency at 12 months: 81.5% all lesion lengths (n=53). This study included LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 20-200 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD-23527v2
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
- Based on physician ratings during animal testing. May not be indicative of clinical performance. Data on file at Bard Peripheral Vascular, Inc., Tempe, AZ.
- The GeoAlign™ Marking System provides an approximation that may not be an exact representation of the distance traveled intravascularly and should be confirmed under fluoroscopy.
- Freedom from TLR at 3 years: 75.5% LifeStent™ Vascular Stent arm (n=134), 41.8% PTA arm (n=72), p LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 40-80 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
- Primary Patency at 2 years: 64.2% LifeStent™ Vascular Stent arm (n=89), 31.3% PTA arm (n=94), p=0.0001. Patency rates calculated when provisional stenting is considered TLR. Kaplan-Meier analysis with Mantel-Cox log-rank test. The study included LifeStent™ Vascular Stent in 6 mm, 7 mm and 8 mm diameters and lengths of 20-170 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
- Primary Patency at 12 months: 81.5% all lesion lengths (n=53). This study included LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 20-200 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
- RESILIENT I/II Trials, E-TAGIUSS Trial, STELLA Trial, Retrospective Analysis of LifeStent™ Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent™ Vascular Stent Delivery System Study (LifeStent™ 200 mm Trial), CONTINUUM Trial, REALITY I/II/III Trials, ETAP Trial, and RELIABLE Trial.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.