
Quality is the foundation of customer and patient trust; it is at the core of what we do to provide safe, effective products and services. All BD associates are committed to a culture of quality inspired by the customers and patients we serve.
In BD Medical-Pharmaceutical Systems (BDM-PS), we have standardized quality management systems and organizational tools across our global network to ensure consistent quality and reporting and facilitate customer audits and site validations.
Our teams have:
- expertise in regional and local healthcare needs
- knowledge of regional and local manufacturing requirements
- mastery of key technical steps to prevent recall risk
- established a global and harmonized manufacturing process that meets stringent standards and delivers products that meet state-of-art requirements.
We stay up to date on manufacturing requirements and have been aware of the EU GMP Annex 1 update since it was communicated in 2017. BDM-PS actively contributed to this revision by providing comments during the first consultation in December of that year and the second consultation in December 2019. The EU GMP Annex 1: 2022 revision was published in August 2022 and became effective on August 25, 2023.
The EU GMP Annex 1: 2022 revision provides general Good Manufacturing Practices guidelines for Pharmaceutical Companies to ensure that microbial, particulate and endotoxin/pyrogen contamination is prevented in the final product for patient safety.
As the primary packaging supplier for pharmaceutical companies, BDM-PS products shall be safe and effective and shall not compromise the clinical condition or safety of patients. As part of our continuous improvement, we apply a risk-based approach to improve our practices and to support the success of pharmaceutical companies with integrating the requirements of EU GMP Annex 1: 2022 revision. This approach includes strategies based on following key elements, as described in the EU GMP Annex 1:
- implementation of a contamination control strategy with a holistic approach
- application of quality risk management principles in decision making
- the use of new technologies.
At BD, our quality policy is to consistently provide superior products and services in pursuit of our purpose of advancing the world of health™. This will be achieved through customer-focused continuous improvement and maintaining an effective quality system which complies with regulatory requirements.
Explore our products
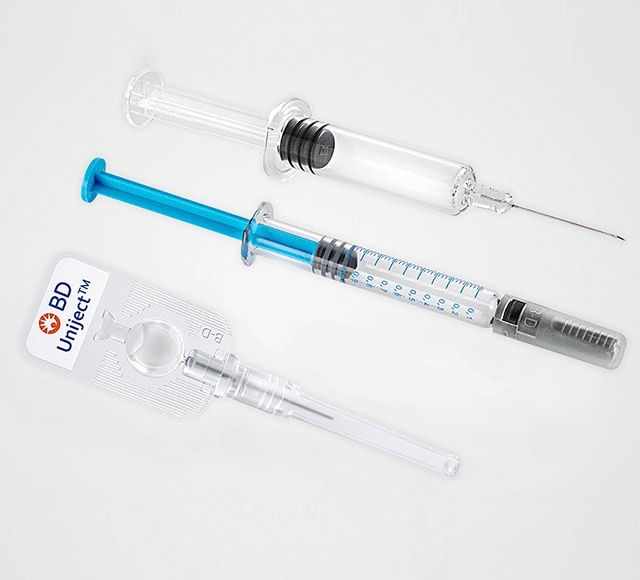
Prefillable syringe systems
BD is uniquely positioned to offer prefillable syringe systems with expertise in drug container interactions, primary container selection and container/device integration for various drug therapies.
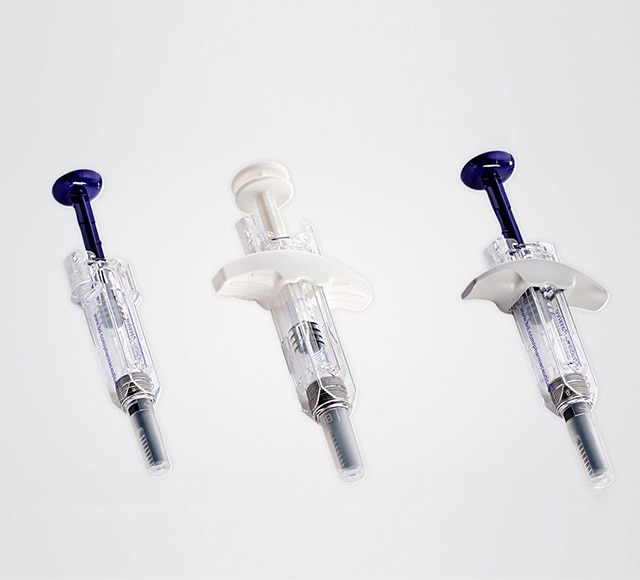
Safety and shielding systems
BD offers a wide range of safety and shielding systems that feature innovative needle shielding system technology for your injectable drug.
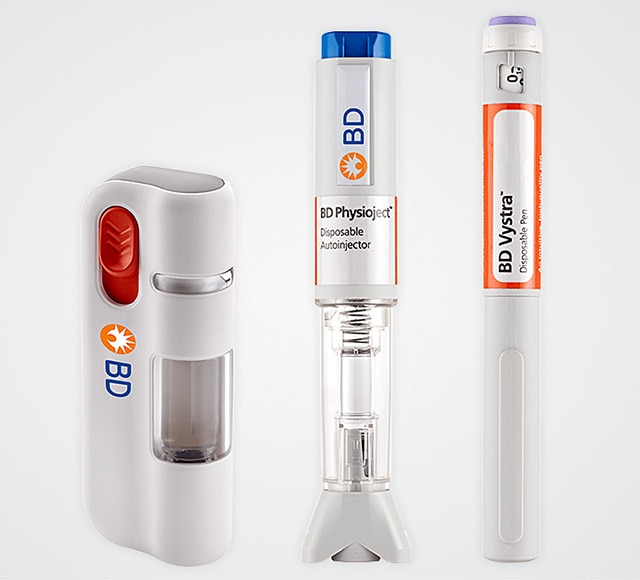
Self injection systems
BD partners with you to develop self-injection systems that enable drug administration across a range of volumes and viscosities, leveraging BD primary container technologies and expertise with a focus on reaching the market faster.

Add ons and components
To fulfill our system approach, we provide a complete set of components for your pre-fillable syringe systems.
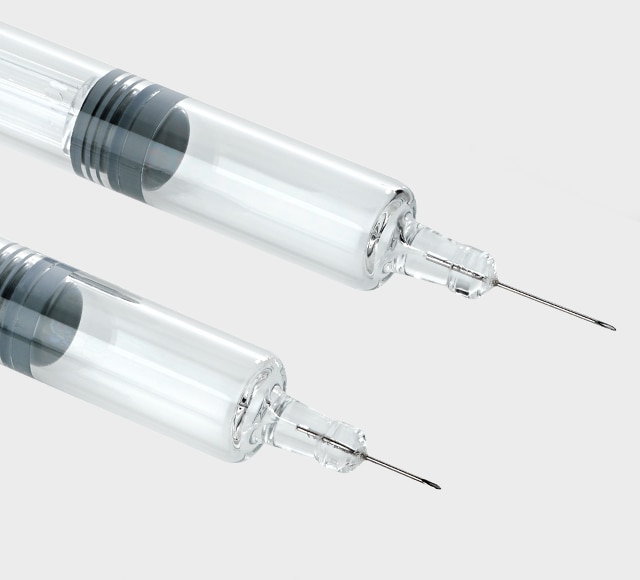
Needle technologies
BD uses proprietary needle technologies to develop needles that optimize your drug delivery.