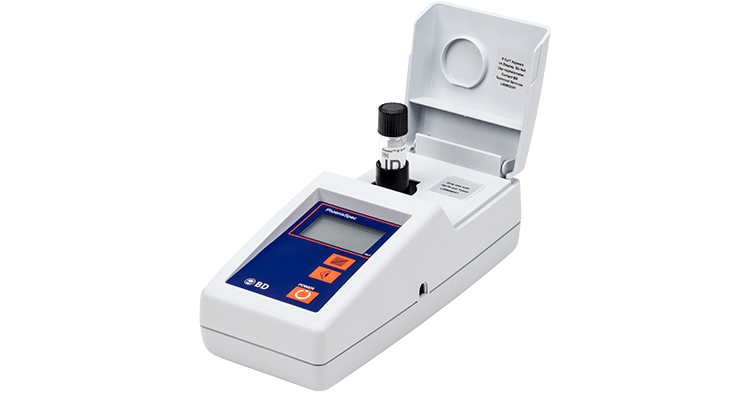
The BD PhoenixSpec™ nephelometer is a portable device designed to measure the turbidity of microbial suspensions equivalent to McFarland standards of 0.10 to 4.50. The instrument may be used to measure the inoculum density for the BD BBL™ Crystal™ identification system and the BD Phoenix™ automated identification and susceptibility testing system.
true
Support
Singapore (Greater Asia Head Office)
+65 6664 2700
bd_sea@bd.com
Contact Us
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Address
2 International Business Park Road, #08-08 The Strategy, Singapore 609930


- Overview
- Products & Accessories
- EIFU & Resources
true
false
true
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
true
false
true

