Bard® Soft Mesh Pre-shaped
Large pore monofilament polypropylene mesh that has a soft, compliant knit structure.

- Overview
- Products & Accessories
- EIFU & Resources
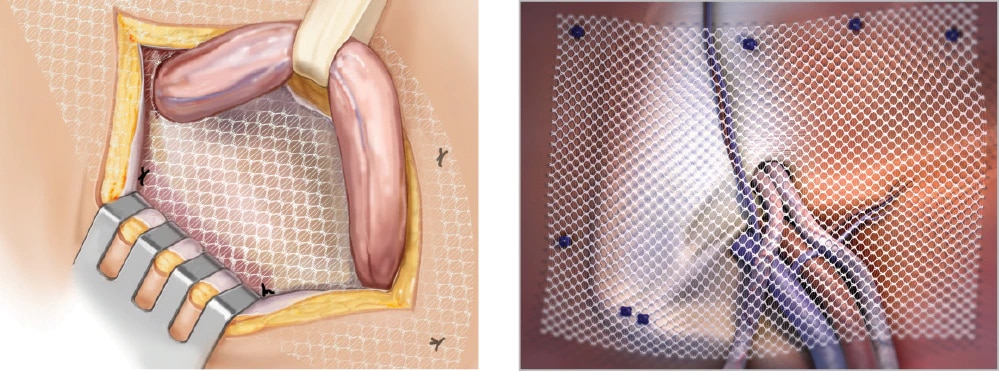
Lightweight mesh designed for open and laparoscopic hernia repair.
Bard® Soft Mesh Pre-shaped offers flexibility, smooth rounded corners and a large pore knit structure which does not lend itself to unraveling or fraying when cut. For surgeons who may prefer to implant less material, it is approximately 60% lighter than traditional polypropylene mesh, and features a soft, thin knit structure that facilitates mesh positioning.
* As compared to other traditional polypropylene mesh, such as Classic Bard® Mesh
* As compared to other traditional polypropylene mesh, such as Classic Bard® Mesh
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
* As compared to other traditional polypropylene mesh, such as Classic Bard® Mesh
