Ready to learn more? Let’s have a conversation.
- Overview
- Recognizing the Risks
- Guideline Support & Education
- Related Products
Solutions you can count on
You shouldn’t have to compromise on hazardous drug safety.
From the compounding pharmacist to the nurse who administers treatment to the staff cleaning a facility, an exposure can occur at almost any moment during the hazardous drug journey. The risk of hazardous drug exposure to healthcare workers is real and significant.
There’s no room for compromise.
With evidence-based insights on your current safe handling practices, rapid hazardous drug detection and closed-system drug transfer device (CSTD) solutions—all designed to support alignment with industry guidelines—the BD® Hazardous Drug Solutions portfolio is designed to help protect you—and the team you count on.
Solutions with demonstrated impact
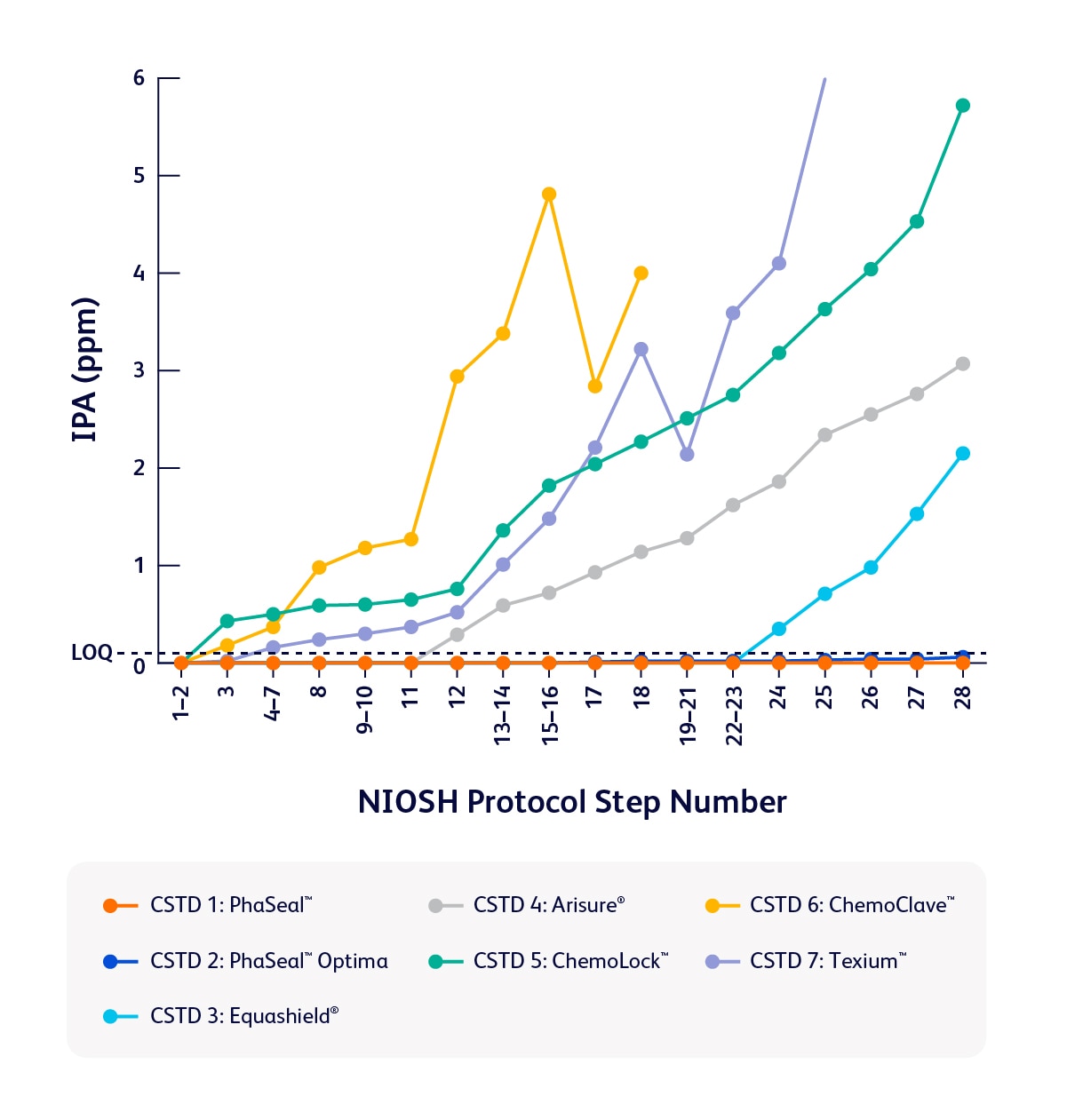
A study published in the Journal of Oncology Pharmacy Practice revealed that of seven tested, the PhaSeal™ Family of CSTDs performed the best in the study to contain vapors during preparation and administration tasks—delivering reliable performance when it matters most.1
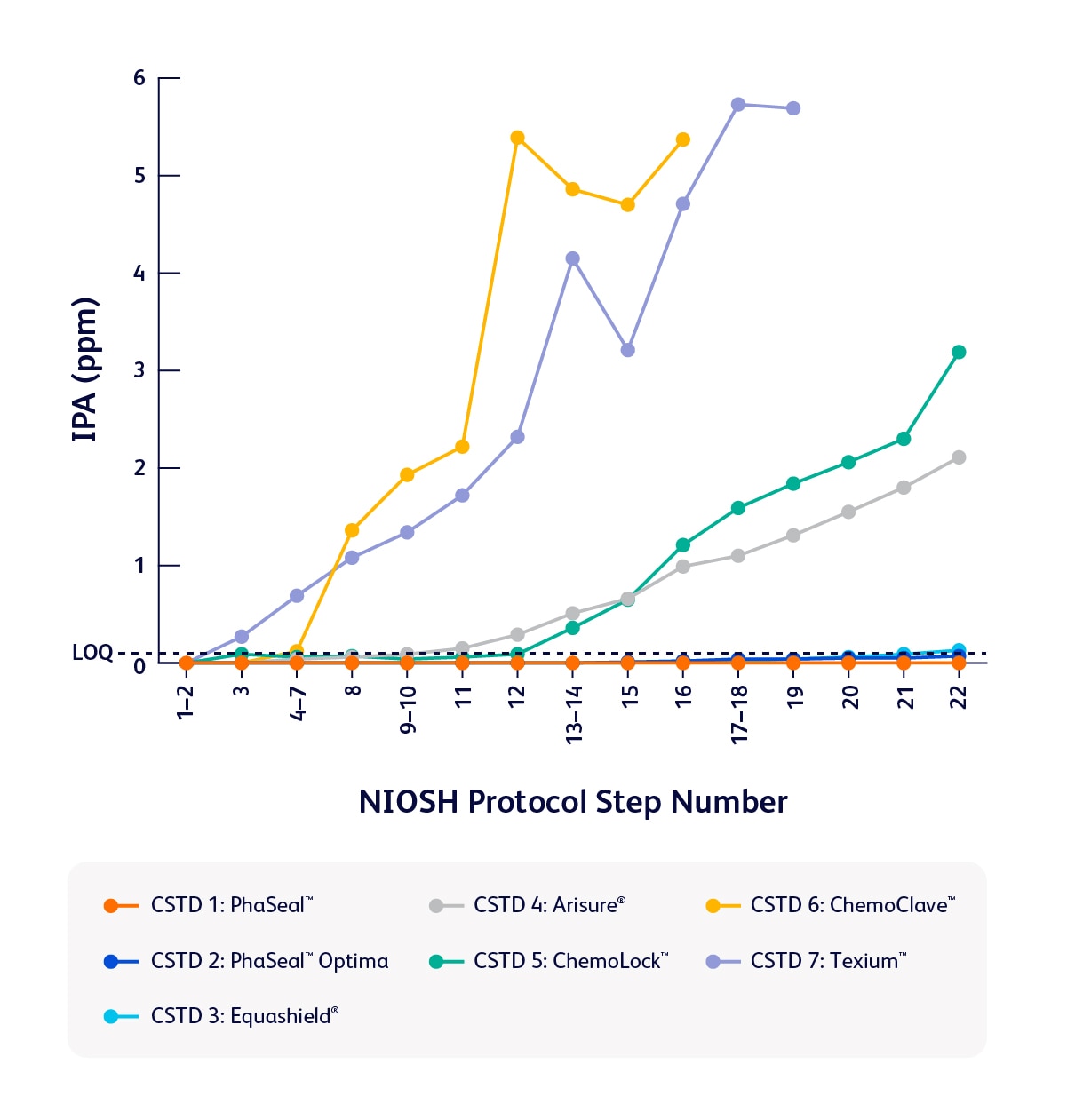
Comparison of task 1 results
A study published in the American Journal of Health-System Pharmacy (AJHP) highlighted the value of integrating PhaSeal™ Optima System and BD® HD Check System into routine clinical practice.2
-

46% reduction in hazardous drug contamination after incorporating BD PhaSeal™ Optima System into clinical workflows1
-

Of the 5 hazardous drugs that showed pretest contamination at one testing site, 4 showed fewer contaminated surfaces post-test, and ALL showed reduced post-test contamination concentrations1
-

As part of a multifaceted approach, the PhaSeal™ Optima System and BD HD Check System may help minimize barriers to routine monitoring, ultimately improving the safety of healthcare workers and patients2
Ready to advance your facility’s safe handling practices? Let’s get started.
- Armistead LT, Stepanovic M, Earnhart MA, Eckel SF. An assessment of seven closed system transfer devices in accordance with the 2015 NIOSH vapor containment performance protocol. J Oncol Pharm Pract. Published online December 10, 2024. doi:10.1177/10781552241304638.
- Brechtelsbauer E. Identification and reduction of hazardous drug surface contamination through the use of a novel closed-system transfer device coupled with a point-of-care hazardous drug detection system. Am J Health Syst Pharm. 2023;80(7):435-444. doi:10.1093/ajhp/zxac336.
Widespread,
well-documented risk
Every year, more than 8 million healthcare workers face potential exposure to hazardous drugs.
From pharmacists and nurses to environmental services and shipping staff, the risk extends across the entire medication journey.1 While these drugs are essential for patient care, their presence in the workplace demands a higher standard of safety.
Potential for serious consequences
The risks of hazardous drug exposure are real—and potentially life-altering
Chromosomal aberrations
A 2.5-fold to 5-fold increase in total chromosomal aberrations has been found among healthcare workers handling hazardous drugs2-4

Organ damage
Nurses handling antineoplastic drugs have an increased risk of liver damage7

Cancer risks
A 1.5-fold increase in nonmelanoma skin cancer and 3.7-fold increase in non-Hodgkin lymphoma has been observed among pharmacy techs5
Nurses exposed to hazardous drugs face a 10.65-fold higher risk of leukemia compared to those without exposure6

Reproductive issues
Staff handling antineoplastic drugs face 2x the risk of miscarriage,8 along with an increased risk of birth defects in their children9
Exposure to hazardous drugs may also present a reproductive risk for men and women actively trying to conceive10
Exposure can occur anywhere on the HD journey
The risk of hazardous drug exposure isn’t confined to a single step. Exposure may occur anywhere hazardous drugs are present in your facility—from pharmacy receiving and preparation to administration and disposal.
A study of six hospitals found measurable levels of hazardous drug contamination on frequently contacted surfaces at every stage of the hospital medication system.11
It’s time to assess and advance your facility’s safe handling practices. BD can help.
- Centers for Disease Control and Prevention (CDC). Hazardous drug exposures in health care. The National Institute for Occupational Safety and Health (NIOSH) Web site. http://www.cdc.gov/niosh/topics/hazdrug. Accessed April 11, 2023.
- Cavallo D, Ursini CL, Perniconi B, et al. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat Res. 2005;587(1-2):45–51. doi:10.1016/j.mrgentox.2005.07.008.
- McDiarmid MA, Oliver MS, Roth TS, Rogers B, Escalante C. Chromosome 5 and 7 abnormalities in oncology personnel handling anticancer drugs. J Occup Environ Med. 2010;52(10):1028–34. doi:10.1097/JOM.0b013e3181f73ae6.
- Roussel C, Witt KL, Shaw PB, Connor TH. Meta-analysis of chromosomal aberrations as a biomarker of exposure in healthcare workers occupationally exposed to antineoplastic drugs. Mutat Res. 2019;781:207-217
- Hansen J, Olsen JH. Cancer morbidity among Danish female pharmacy technicians. Scand J Work Environ Health. 1994;20(1):22–26. doi:10.5271/sjweh.1433.
- Skov T, Maarup B, Olsen J, et al. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs. Br J Ind Med. 1992;49(12):855–861. doi:10.1136/oem.49.12.855.
- Sotaniemi EA, Sutinen S, Arranto AJ, et al. Liver damage in nurses handling cytostatic agents. Acta Med Scand. 1983;214(3):181–189. doi:10.1111/j.0954-6820.1983.tb08593.x.
- Lawson CC, Rocheleau CM, Whelan EA, et al. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol. 2012;206(4):327.e1-8.
- Hemminki K, Kyronen P, Lindbohm ML. Spontaneous abortions and malformations in the offspring of nurses exposed to anaesthetic gases, cytostatic drugs, and other potential hazards in hospitals, based on registered information of outcome. J Epidemiol Community Health. 1985;39(2):141–147. doi:10.1136/jech.39.2.141.
- National Institute for Occupational Safety and Health (NIOSH). Current intelligence bulletin 68: NIOSH chemical carcinogen policy. By Whittaker C, Rice F, McKernan L, Dankovic D, Lentz TJ, MacMahon K, Kuempel E, Zumwalde R, Schulte P, on behalf of the NIOSH Carcinogen and RELs Policy Update Committee. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2017‐100. https://www.cdc.gov/niosh/docs/2017‐100/pdf/2017‐100.pdf. Accessed March 20, 2020.
- Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg. 2013;10(7):374–383. doi:10.1080/15459624.2013.789743.
Is your facility up to date with current best practices?
For decades, clinical organizations have provided evidence-based recommendations to help protect healthcare workers from the risks of hazardous drug exposure. As protective technology and knowledge advance, these guidelines evolve. Staying current is a critical part of keeping your team safe.
Closed-system drug-transfer device (CSTD) usage guidelines
The use of CSTDs has been widely recommended by different organizations, including the United States Pharmacopeial Convention (USP),1 American Society of Health-System Pharmacists (ASHP),2 National Institute for Occupational Safety and Health (NIOSH),3 Oncology Nursing Society,4 National Association of Pharmacy Regulatory Authority (NAPRA),9 and Canadian Vascular Access & Infusion Therapy (CVAA).10
“CSTDs should be used when compounding HDs when dosage form allows.”
“CSTDs must be used for administration of antineoplastic HDs when the dosage form allows.”
“Use and maintenance of proper engineering controls (e.g., C-PECs, C-SECs, and CSTDs).”
“HDs must be administered safely using protective medical devices and techniques, examples of protective medical devices include needleless and closed systems.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Consider using devices such as closed-system transfer devices, glove bags, and needleless systems when transferring hazardous drugs from primary packaging (such as vials) to dosing equipment (such as infusion bags, bottles, or pumps).”
“Develop workplace procedures for using and maintaining all equipment that functions to reduce exposure—such as ventilated cabinets, closed-system drug-transfer devices, needle-less systems, and PPE.”
— NIOSH alert 2004: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings.3
“For these reasons, USP chapter 800 has determined that CSTDs should be used when compounding HDs and that CSTDs must be used when administering antineoplastic HDs when the dosage form allows and the device is physically or chemically compatible with the HD to be used.”
— ASHP Guidelines on Handling Hazardous Drugs2
"Use a Closed System Transfer Device (CSTD) for compounding, preparation, and administration to minimize HCP and patient exposure to hazardous medication (INS, 2024)"
— CVAA Guidelines 2024, Core Practice Principles — Safe Handling and Disposal of Hazardous Materials and Sharps, pg. 2410
“Safely administer antineoplastics in accordance with organizational and regulatory guidance... Use Closed System Transfer Device (CSTD) system to reduce risk of droplet and aerosolized medication exposure. A CSTD should be used at every connection point with IV push/direct and IV infusions (INS, 2024)"
— CVAA Guidelines 2024, Anti-neoplastic Therapy — Administration, pg. 8610
"Compounding personnel must adhere to the following requirements when working inside the C-PEC: ...If possible, use a closed-transfer system (since the steps described above do not completely eliminate the risk of exposure to the hazardous preparation)."
— NAPRA Guidelines for Pharmacy Compounding of Non-Sterile Preparations 2016 (Aseptic technique for compounding hazardous sterile preparations - 6.6.5.2)9
Surface contamination monitoring guidelines
Organizations, health agencies and conferences around the world, such as the USP,1 ASHP2 and NAPRA,9 recommend routine monitoring as part of a comprehensive safe handling program.
"The level of hazardous product contamination should be measured at least once every 6 months, more frequently if there has been a major change in placement of furniture, compounding processes or cleaning practices. Sampling should be performed at the various sites used for compounding, especially those most likely to be contaminated (e.g., outside the C-PEC, floor surrounding the C-PEC)."
— NAPRA Guidelines for Pharmacy Compounding of Non-Sterile Preparations 2016 (9.6.3 Hazardous product contamination and wipe sampling)9
“Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every six months, or more often as needed, to verify containment). […] Repeat the wipe sampling to validate that the deactivation/decontamination and cleaning steps have been effective.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Surface wipe sampling of healthcare settings for HD contamination is advocated as a means of environmental quality and control. Surface wipe sampling should be done routinely, first to determine a benchmark of contamination and then to monitor the effectiveness of safe handling programs.”
— ASHP Guidelines on Handling Hazardous Drugs2
“Each element of the monitoring program must be included in a sampling plan with sample locations, methods of collection, sampling frequency, and other specifics depending on the type of monitoring being performed.”
“Records of data collected through the monitoring program must be maintained as part of the overall quality assurance program of the facility.”
“Ensuring a safe compounding environment requires viable and nonviable airborne particle testing, pressure differential or displacement airflow measurement, temperature monitoring, and surface disinfection sampling and assessment. Each element…must be included in a sampling plan with sample locations, methods of collection, sampling frequency, and other specifics depending on the type of monitoring being performed.”
— ASHP Guidelines on Compounding Sterile Preparations5
Recommended sampling locations
Standards and guidelines have identified several locations in pharmacy and patient administration areas where routine wipe sampling for HD surface contamination might be beneficial.
test the section name text
test the section description text

test the section name text
test the section description text

Training and education recommendations
Comprehensive safe handling programs include comprehensive training and routine reassessments. Both the USP1 and Oncology Nursing Society4 provide specific recommendations for annual HD education.
“All personnel who handle HDs must be trained based on job functions… Personal competency must be reassessed every 12 months.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Comprehensive didactic education and documentation of clinical competence is required for all HCW and must be reassessed at least every 12 months.”
— Oncology Nursing Society—Safe Handling of Hazardous Drugs4
Ready to find out how up to date your facility is with guidelines and best practices?
- United States Pharmacopeial Convention. USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare. Published February 1, 2016. Updated June 26, 2020. Accessed April 11, 2023.
- Power LA, Coyne JW. ASHP Guidelines on Handling Hazardous Drugs. Am J Health Syst Pharm. 2018;75(24):1996-2031. doi:10.2146/ajhp180564
- National Institute for Occupational Safety and Health (NIOSH). NIOSH alert 2004: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf. Accessed August April 11, 2023.
- Polovich M, Olsen, M eds. Safe Handling of Hazardous Drugs. 3rd ed. Pittsburgh, PA: Oncology Nursing Society; 2018.
- American Society of Health-System Pharmacists (ASHP). ASHP guidelines on compounding sterile preparations. Am J Health Syst Pharm. 2014;71(2):145-166. Accessed June 10, 2025. https://www.ashp.org/-/media/assets/policy-guidelines/docs/guidelines/compounding-sterile-preparations.ashx
- Valero-García S, González-Haba E, Gorgas-Torner MQ, et al. Monitoring contamination of hazardous drug compounding surfaces at hospital pharmacy departments. A consensus Statement. Practice guidelines of the Spanish Society of Hospital Pharmacists (SEFH). Farm Hosp. 2021;45(2):96–107. doi:10.7399/fh.11655.
- Gabay M, Johnson P, Fanikos J, et al. Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. Am J Health Syst Pharm. 2021;zxab134. doi:10.1093/ajhp/zxab134.
- Domingo T, Fontán G, Enríquez M, et al. Guía monitorización de superficies de medicamentos peligrosos. 1. ed. Instituto Español de Investigación Enfermera; Consejo General de Enfermería; 2021.
- National Association of Pharmacy Regulatory Authorities (NAPRA). Model standards for Pharmacy Compounding of Non-Hazardous Sterile Preparations. Ottawa, ON: NAPRA; 2016. Available from: https://www.napra.ca/wp-content/uploads/2024/03/NAPRA-Mdl-Stnds-Pharmacy-Compounding-Hazardous-Sterile-Preparations-Sep-2016-Revised-b.pdf
- Guidelines Development Group on behalf of Canadian Vascular Access Association.(2024). Canadian Vascular Access and Infusion Therapy Guidelines, 2nd Edition. Pappin Communications.
Related Products
-

The first rapid hazardous drug detection* system that provides easyto- read binary results in less than 10 minutes to help facilitate routine monitoring and evaluate your institution’s safe handling practices.
*The BD® HD Check System tests for select hazardous drugs— cyclophosphamide, doxorubicin and methotrexate. Surfaces with contamination at or above the limits of detection have 95% specificity and sensitivity. -
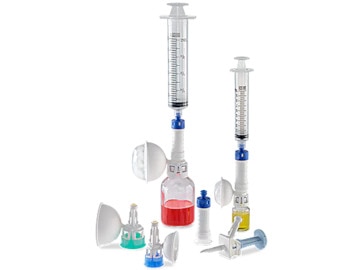
The BD PhaSeal™ System pioneered the category of CSTDs to help protect the healthcare workers who prepare and administer hazardous drugs. Twenty years later, we turned to healthcare professionals like you for feedback and guidance to optimize its every component. The result is the BD PhaSeal™ Optima System—a next-generation, airtight and leakproof CSTD solution designed to maximize drug extraction*† while advancing safety.
*Compared to similar systems
†Bench test results may not necessarily be indicative of clinical performance -
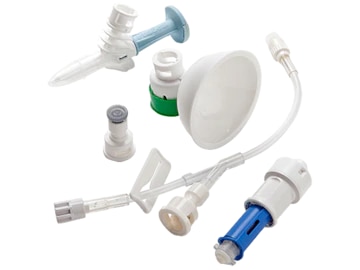
The BD PhaSeal System is airtight and leakproof to protect your staff from hazardous drug exposure during drug preparation, administration and disposal.