Giving you more control in three key ways
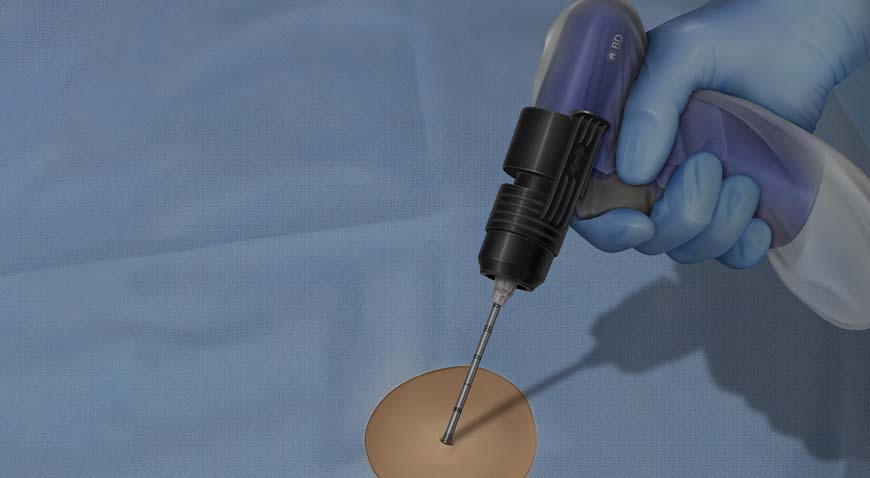
The BD Trek™ Powered Bone Biopsy System's variable speed driver design places control at your fingertips in three key ways:
- Lower Speed Control allows for precision when gaining access or when targeting challenging areas in small or round bones
- Higher Speed Control when power is needed, such as collecting a core or penetrating sclerotic bone
- Automatic Brake Control stops the needle rotation automatically upon trigger release