-
Halo One™ Thin-Walled Guiding Sheath 25CM, 6 French, 0.035” guidewire
SKU/REF hlo62535h
-
Halo One™ Thin-Walled Guiding Sheath 10CM, 6 French, 0.035” guidewire
SKU/REF hlo61035fh
true

- Overview
- Products & Accessories
- EIFU & Resources
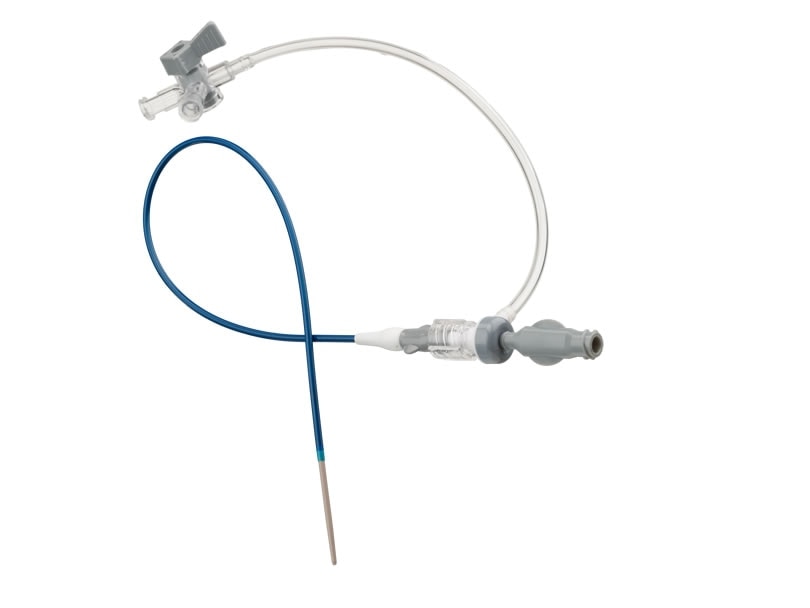
The Halo One™ Thin-Walled Guiding Sheath is designed to perform as both a guiding sheath and an introducer sheath.
The Halo One™ Sheath provides a 1 French wall thickness which reduces the size of the arteriotomy compared to standard sheaths of the same French size without sacrificing inner diameter. Reinforced by a stainless-steel braid, the shaft construction is designed to provide the flexibility, tensile strength, and kink resistance needed when navigating tortuous anatomies. A robust size offering enables treatment from various approaches including femoral, pedal, and radial access sites.
- Low profile design reduces arteriotomy size which can help to minimize access site complications1
- Only thin-walled guiding sheath with lengths suitable for distal peripheral intervention2
- Stainless-steel reinforced shaft construction is designed to perform even in challenging anatomy
Made for today, tomorrow and for what’s needed next.
1 Ortiz, Daniel, et al. “Access site complications after peripheral vascular interventions: incidence, predictors, and outcomes.” Circulation: Cardiovascular Interventions 7.6 (2014): 821-828.
2 As of December 2021 on the U.S. market.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
1 Ortiz, Daniel, et al. “Access site complications after peripheral vascular interventions: incidence, predictors, and outcomes.” Circulation: Cardiovascular Interventions 7.6 (2014): 821-828.
2 Shaft lengths of 45, 70, and 90cm are available in 4F and 5F sizes only.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
true
true
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Training
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
Learn More
Case Studies
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
1 Ortiz, Daniel, et al. “Access site complications after peripheral vascular interventions: incidence, predictors, and outcomes.” Circulation: Cardiovascular Interventions 7.6 (2014): 821-828.
2 Shaft lengths of 45, 70, and 90cm are available in 4F and 5F sizes only.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
true
true