true

- Overview
- Products & Accessories
- EIFU & Resources
- Kit Components
- FAQ
- Available in 12 French (Fr.) and 13 French (Fr.)
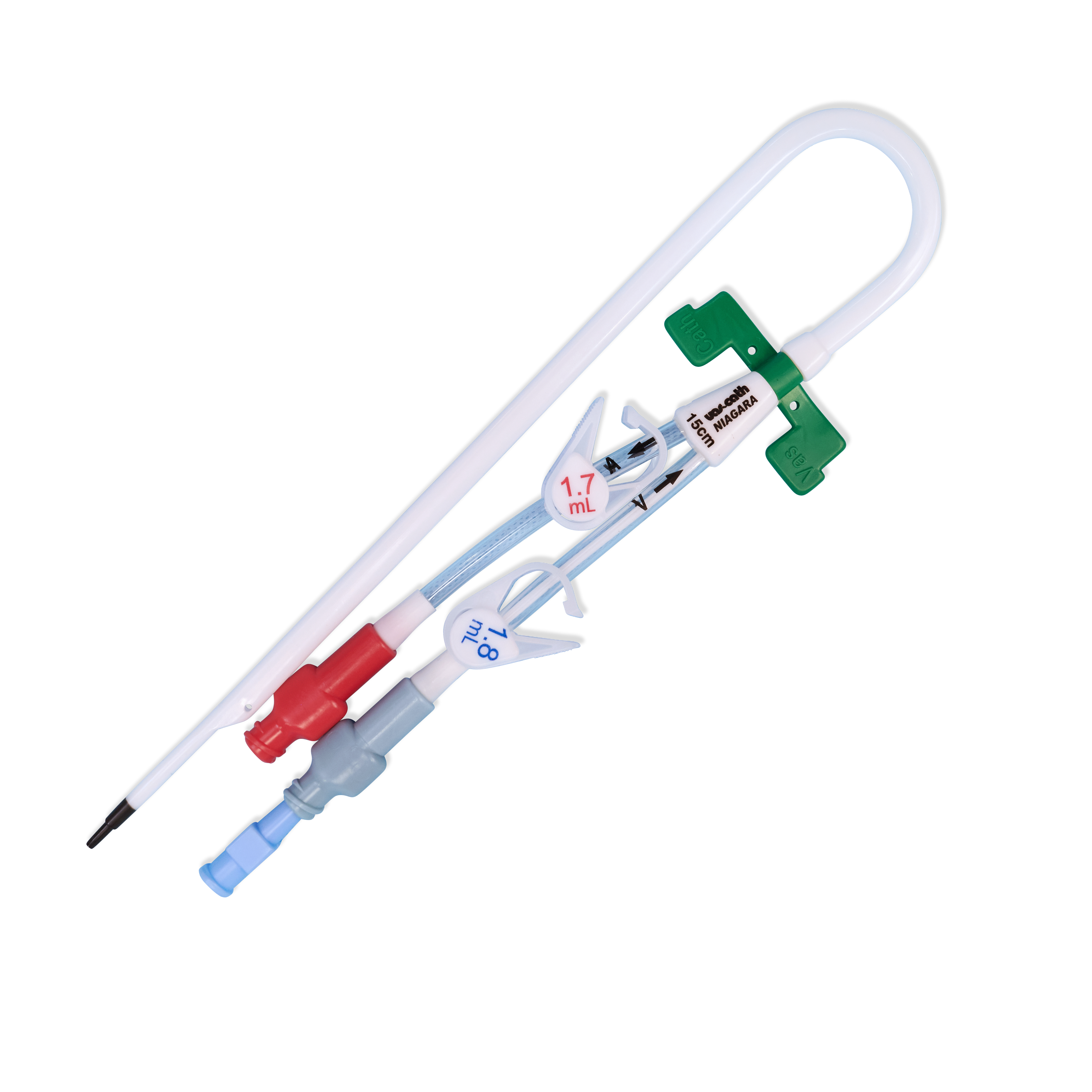
- Double Barrel Lumen Design
- Kink Resistant Catheter Design
- Step Tip
- Catheter Length Options
- Catheter or Kit Options
- Maximal Battier Trays
- Straight or pre-curved cathete
Start Acute Dialysis Care
The Niagara™ (13 Fr.) and Niagara™ Slim-Cath™ (12 Fr.) Short-Term Dual Lumen Dialysis Catheter is an easy to place, kink resistant, dual-lumen catheter designed for flow rates of 400/mL/min with pressures of less than 250 mmHg* . The Niagara™ Slim-Cath™ (12 Fr.) delivers flow rates of 300 mL/min. Both the 13 Fr. and 12 Fr. catheters demonstrate less than 2% recirculation rate on average when lines are run in forward and reverse. Note that recirculation in femoral placed catheters is likely significantly greater than in internal jugular placed catheters.
[AN1]Excludes Niagara Slim-Cath for 400mL/min with pressures of less than 250 mmHg.
Optimal Performance Features
- Double Barrel Lumen Design: allows for flows of 400 mL/min in dialysis lumens with pressures of less than 250 mmHg
- Kink Resistant Catheter Design: resists kinking while providing stiffness for insertion
- Step Tip: At a flow rate of 400 mL/min (Niagara™) 300 mL/min (Niagara™ Slim-Cath™) the step tip design demonstrated less than 2% recirculation on average when lines are run in forward and reverse. Note that recirculation in femoral catheters is likely significantly greater than in internal jugular placed catheters.
- Catheter: Pre-curved catheter options may help improve dressing and accessing extensions to administer treatment
- Tip: is softer than shaft
Benefits of the Niagara™ and Niagara™ Slim-Cath™ Short-Term Dual Lumen Dialysis Catheters
- 13 French Niagara™ and 12 French Niagara™ Slim-Cath™ Short-Term Dual Lumen Catheters are available in a variety of lengths including a long 30 cm length for femoral placements. Catheters are available in two configurations – with straight and curved extension legs – and in standard kits and full maximal barrier precautions trays. Full maximal barrier precautions trays help clinicians comply with CPSI, HC, and CCOHS guidelines and feature a full body drape (70” x 110”), a surgical gown, and safety components.
- FLEXIBILITY and UTILITY for You and Your patient
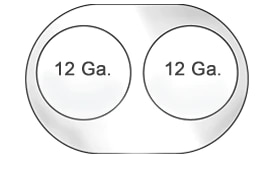
- Dialysis (12 Ga) Lumens indicated for hemodialysis, hemoperfusion, and apheresis
- Catheter Length Options catheters are available in 12.5 cm, 15 cm, 24 cm, and 30 cm (lengths vary by catheter configuration)
Design
- Flow Rate of 400 ml/min (Niagara™) and 300 mL/ (Niagara™ Slim-Cath™) with pressures of less than 250 mmHg in dialysis lumens
- Intra lumens for Niagara™ are 12 Ga and 11 Ga for the Niagara™ Slim-Cath™
- Double Barrel Lumen Design with round intra lumens
Kink Characteristics
- 12 Fr. and 13 Fr. Oval Catheter Shaft designed for excellent kink resistance
- More Kink Resistant than competitive 11.5 Fr. dual lumen dialysis catheter
Maximal Barrier Trays
Feature a full body drape (70" x 110"), surgical gown, and safety components.
Maximal barrier precautions help you comply with the latest guidelines from:
- Health Canada (HC) Guidelines
- Canadian Patient Safety Institute’s (CPSI) Safer Healthcare Now!
- ·Canadian Centre for Occupational Health and Safety (CCOHS) Guidelines
true
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Training
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
Learn More
Case Studies
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
| Description | Tray (Catheter) | Kit |
| Niagara™/Niagara™ Slim-Cath™ Cath Catheter | 1 | 1 |
| Dualator™ Vessel Dilator 11-13 Fr. | 1 | 1 |
| Dualator™ Vessel Dilator 12-14 Fr. | 1 | 1 |
| Guidewire Introducer Needle | 0 | 1 |
| Guidewire 70 cm x 0.97 mm (0.038 in.) | 0 | 1 |
| Transparent Dressing | 0 | 1 |
| Attachable Suture Wing.) | 1 | 1 |
| Injection Cap | 2 | 2 |
The Niagara™ and Niagara™ Slim-Cath™ Catheters (Dual Lumen) are indicated for use in attaining short term (less than 30 days) vascular access for hemodialysis, hemoperfusion and apheresis therapy via the jugular, subclavian or femoral vein.
The catheter is intended for short-term vascular access only and is not to be used for any purpose other than indicated. The catheter must not be left in the femoral vein longer than three days. To maintain peak performance, it is recommended that subclavian and jugular catheters be replaced after four weeks.
The Niagara™ and Niagara™ Slim-Cath™ Catheter connectors for the dialysis lumens (blue and red) are made from Delrin™ polyacetal, the extension legs from Tecothane™ polyurethane, and the catheter shaft is made from thermosensitive Bodysoft®polyurethane which softens at body temperature. The product and packaging do not contain natural rubber latex.
false
The Niagara™ and Niagara™ Slim-Cath™ Catheters (Dual Lumen) are indicated for use in attaining short term (less than 30 days) vascular access for hemodialysis, hemoperfusion and apheresis therapy via the jugular, subclavian or femoral vein.
The catheter is intended for short-term vascular access only and is not to be used for any purpose other than indicated. The catheter must not be left in the femoral vein longer than three days. To maintain peak performance, it is recommended that subclavian and jugular catheters be replaced after four weeks.
The Niagara™ and Niagara™ Slim-Cath™ Catheter connectors for the dialysis lumens (blue and red) are made from Delrin™ polyacetal, the extension legs from Tecothane™ polyurethane, and the catheter shaft is made from thermosensitive Bodysoft®polyurethane which softens at body temperature.
The product and packaging do not contain natural rubber latex.
The Niagara™ Catheter flows at 400 mL/min for all lengths with low arterial/venous pressures (less than 250 mmHg). As suggested by in vitro data using a blood simulate approximating the viscosity of whole blood.
The Niagara™ Slim-Cath™ Catheter flows at 300 mL/min for all lengths with low arterial/venous pressures (less than 250 mmHg). As suggested by in vitro data using a blood simulate approximating the viscosity of whole blood.
The rate of recirculation of the dialysis lumens is less than 2% on average in both the forward and reverse directions when tested in vitro1 at a flow rate of 400 mL/min with the step tip design for the Niagara™ Short Term Dialysis Catheter. Note that recirculation in femoral catheters is likely significantly greater than in internal jugular placed catheters.
The rate of recirculation of the dialysis lumens is less than 2% on average in both the forward and reverse directions when tested in vitro1 at a flow rate of 300 mL/min with the step tip design for the Niagara™ Slim-Cath™ Short Term Dialysis Catheter. Note that recirculation in femoral catheters is likely significantly greater than in internal jugular placed catheters.
The Niagara™ Catheter outer diameter is 13 French. The intra lumens are large 12 gauge lumens that allow for flows of 400 mL/min.
The Niagara™ Slim-Cath™ Catheter outer diameter is 12 French. The intra lumens are large 11 gauge lumens that allow for flows of 300 mL/min.
The oval shape provides superior kink resistance compared to a round shaft catheter.
The Niagara™ and Niagara™ Slim-Cath™ Catheters are available in both straight and pre-curved catheter configurations.
The Niagara™ and Niagara™ Slim-Cath™ Catheter is available in 12.5 cm (pre-curved catheter configurations only), 15 cm, 20 cm, 24 cm, and 30 cm (straight catheter configurations only).
The Niagara™ and Niagara™ Slim-Cath™ Catheter are available in both standard kit and catheter trays. Both standard kit and catheter tray configurations offer straight and pre-curved catheter configurations.
Transparent, rotatable wing allows for easy visualization and cleaning of exit site.
Each kit includes an 11-13 Fr. Dualator® Vessel Dilator and a 12-14 Fr. Dualator® Vessel Dilator. Placers should dilate to the largest portion of each Dualator® Vessel Dilator to allow the catheter to more easily enter the tissue and vessel.
*Catheter trays do not contain a guidewire, guidewire introducer needle, and transparent dressing
The Niagara™ and Niagara™ Slim-Cath™ Catheter connectors for the dialysis lumens (blue and red) are made from Delrin™ polyacetal, the extension legs from Tecothane™ polyurethane, and the catheter shaft is made from thermosensitive Bodysoft®polyurethane which softens at body temperature. The product and packaging do not contain natural rubber latex.
The Niagara™ and Niagara™ Slim-Cath™ Catheters (Dual Lumen) are indicated for use in attaining short term (less than 30 days) vascular access for hemodialysis, hemoperfusion and apheresis therapy via the jugular, subclavian or femoral vein.
The Niagara™ and Niagara™ Slim-Cath™ Catheter connectors for the dialysis lumens (blue and red) are made from Delrin™ polyacetal, the extension legs from Tecothane™ polyurethane, and the catheter shaft is made from thermosensitive Bodysoft®polyurethane which softens at body temperature. The product and packaging do not contain natural rubber latex.
The Niagara™ and Niagara™ Slim-Cath™ Dialysis Catheter is compatible with the following agents: Povidone iodine, 0.55% sodium hypochlorite solution, chlorhexidine gluconate 4%, chlorhexidine gluconate 2% solution, dilute aqueous sodium hypochlorite, hydrogen peroxide, bacitracin zinc ointments in petrolatum bases.
WARNING: Acetone and PEG-containing ointments can cause failure of this device and should not be used with polyurethane catheters. Chlorhexidine patches or bacitracin zinc ointments are the preferred alternatives.
Yes. The StatLock Catheter Stabilization Device is compatible and can be ordered separately from Canadian Hospital Specialties (CHS). The part number is DI0122.
Yes, the 24 cm and 30 cm lengths are recommended for femoral placements.
WARNING:The catheter must not be left in the femoral vein longer than three days.
Yes, the Niagara™ and Niagara™ Slim-Cath™ Catheter may be exchanged over a guidewire. The wire included in the kit is a 0.038 inch wire which fits through the venous lumen of the catheter.
- As suggested by in vitro data using blood simulate approximately the viscosity of whole blood.
- Bench test R61545-0. Bench test results may not necessarily be indicative of clinical performance.
Available sizes: 13 Fr.
Please consult product labels, IFU, and package inserts for any indications, contraindications, hazards, warnings, cautions, and instructions for use.
BD-19967 (03/23)
true

.jpg)

