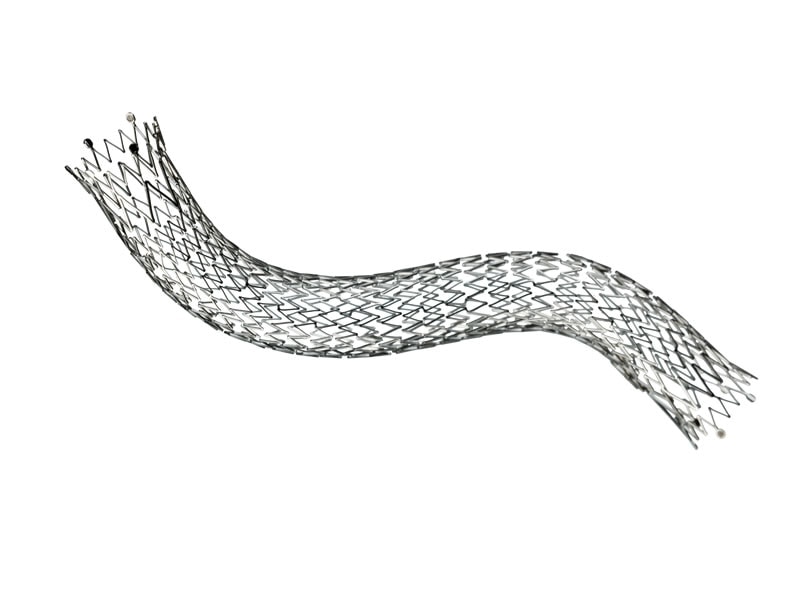
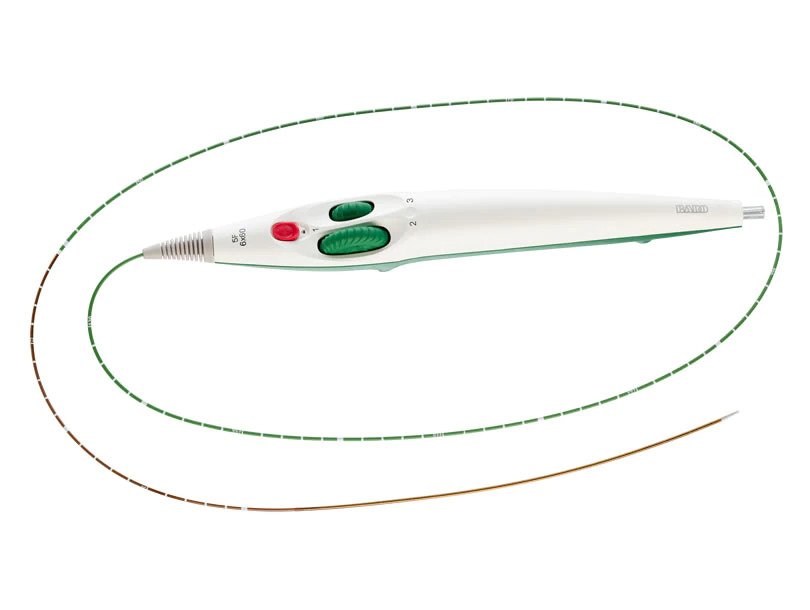
- Unique helical design engineered for bending, compression, torsion
- Low profile, 5F delivery system
- Dual-speed thumbwheel deployment designed for ease of use and placement accuracy1
- GeoAlign™ Marking System designed to increase procedural efficiency and reduce radiation exposure2

- Overview
- Products & Accessories
- EIFU & Resources
The LifeStent™ Vascular Stent has a history of proven performance:
- In a Level 1 RESILIENT study, the LifeStent™ Vascular Stent demonstrated treatment superiority over balloon angioplasty with sustained effectiveness out to 3 years3
- In the investigator initiated, Level 1 ETAP study of the popliteal artery the LifeStent™ Vascular Stent demonstrated double the primary patency rate of PTA out to two years4
- In a clinical assessment of the treatment of long lesions, the LifeStent™ Vascular Stent demonstrated high primary patency at 12 months in lesions up to 240mm5
The LifeStent™ 5F Vascular Stent System offers the same clinically-proven advanced helical stent design as the LifeStent™ Vascular Stent on a low profile, 5F delivery system. The LifeStent™ 5F delivery system offers dual speed thumbwheel deployment with a tri-axial catheter that is designed for ease of use, deployment control, and precise placement accuracy.1
The LifeStent™ 5F Vascular Stent System features the GeoAlign™ Marking System, which is a simple-to-use, non-radiopaque ruler on the catheter shaft. The GeoAlign™ Marking System is featured on all Bard 5F ProSeries™ products. The GeoAlign™ Marking System is designed to facilitate repeatable catheter alignment at the lesion and to increase procedure efficiency by minimizing fluoroscopy exposure.2
- Based on physician ratings during animal testing. May not be indicative of clinical performance. Data on file at Bard Peripheral Vascular, Inc., Tempe, AZ.
- The GeoAlign™ Marking System provides an approximation that may not be an exact representation of the distance traveled intravascularly and should be confirmed under fluoroscopy.
- Freedom from TLR at 3 years: 75.5% LifeStent™ Vascular Stent arm (n=134), 41.8% PTA arm (n=72), p LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 40-80 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
- Primary Patency at 2 years: 64.2% LifeStent™ Vascular Stent arm (n=89), 31.3% PTA arm (n=94), p=0.0001. Patency rates calculated when provisional stenting is considered TLR. Kaplan-Meier analysis with Mantel-Cox log-rank test. The study included LifeStent™ Vascular Stent in 6 mm, 7 mm and 8 mm diameters and lengths of 20-170 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
- Primary Patency at 12 months: 81.5% all lesion lengths (n=53). This study included LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 20-200 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
1. Commercially available as of December 2020
2. Based on physician ratings during animal testing. May not be indicative of clinical performance. Data on file at Bard Peripheral Vascular, Inc., Tempe, AZ.
3. The GeoAlign™ Marking System provides an approximation that may not be an exact representation of the distance traveled intravascularly and should be confirmed under fluoroscopy.
4. Freedom from TLR at 3 years: 75.5% LifeStent™ Vascular Stent arm (n=134), 41.8% PTA arm (n=72), p LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 40-80 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
5. Primary Patency at 2 years: 64.2% LifeStent™ Vascular Stent arm (n=89), 31.3% PTA arm (n=94), p=0.0001. Patency rates calculated when provisional stenting is considered TLR. Kaplan-Meier analysis with Mantel-Cox log-rank test. The study included LifeStent™ Vascular Stent in 6 mm, 7 mm and 8 mm diameters and lengths of 20-170 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
6. Primary Patency at 12 months: 81.5% all lesion lengths (n=53). This study included LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 20-200 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
7. RESILIENT I/II Trials, E-TAGIUSS Trial, STELLA Trial, Retrospective Analysis of LifeStent™ Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent™ Vascular Stent Delivery System Study (LifeStent™ 200 mm Trial), CONTINUUM Trial, REALITY I/II/III Trials, ETAP Trial, and RELIABLE Trial.
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
1. Commercially available as of December 2020
2. Based on physician ratings during animal testing. May not be indicative of clinical performance. Data on file at Bard Peripheral Vascular, Inc., Tempe, AZ.
3. The GeoAlign™ Marking System provides an approximation that may not be an exact representation of the distance traveled intravascularly and should be confirmed under fluoroscopy.
4. Freedom from TLR at 3 years: 75.5% LifeStent™ Vascular Stent arm (n=134), 41.8% PTA arm (n=72), p LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 40-80 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
5. Primary Patency at 2 years: 64.2% LifeStent™ Vascular Stent arm (n=89), 31.3% PTA arm (n=94), p=0.0001. Patency rates calculated when provisional stenting is considered TLR. Kaplan-Meier analysis with Mantel-Cox log-rank test. The study included LifeStent™ Vascular Stent in 6 mm, 7 mm and 8 mm diameters and lengths of 20-170 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
6. Primary Patency at 12 months: 81.5% all lesion lengths (n=53). This study included LifeStent™ Vascular Stent in 6 mm and 7 mm diameters and lengths of 20-200 mm. The LifeStent™ 5 mm stent diameter and LifeStent™ 5F delivery system were not included in these clinical studies.
7. RESILIENT I/II Trials, E-TAGIUSS Trial, STELLA Trial, Retrospective Analysis of LifeStent™ Vascular Stent Systems in the Treatment of Long-Segment Lesions, LifeStent™ Vascular Stent Delivery System Study (LifeStent™ 200 mm Trial), CONTINUUM Trial, REALITY I/II/III Trials, ETAP Trial, and RELIABLE Trial.
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.