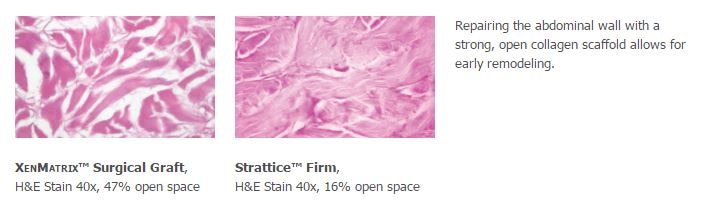
Confidence Reinforced
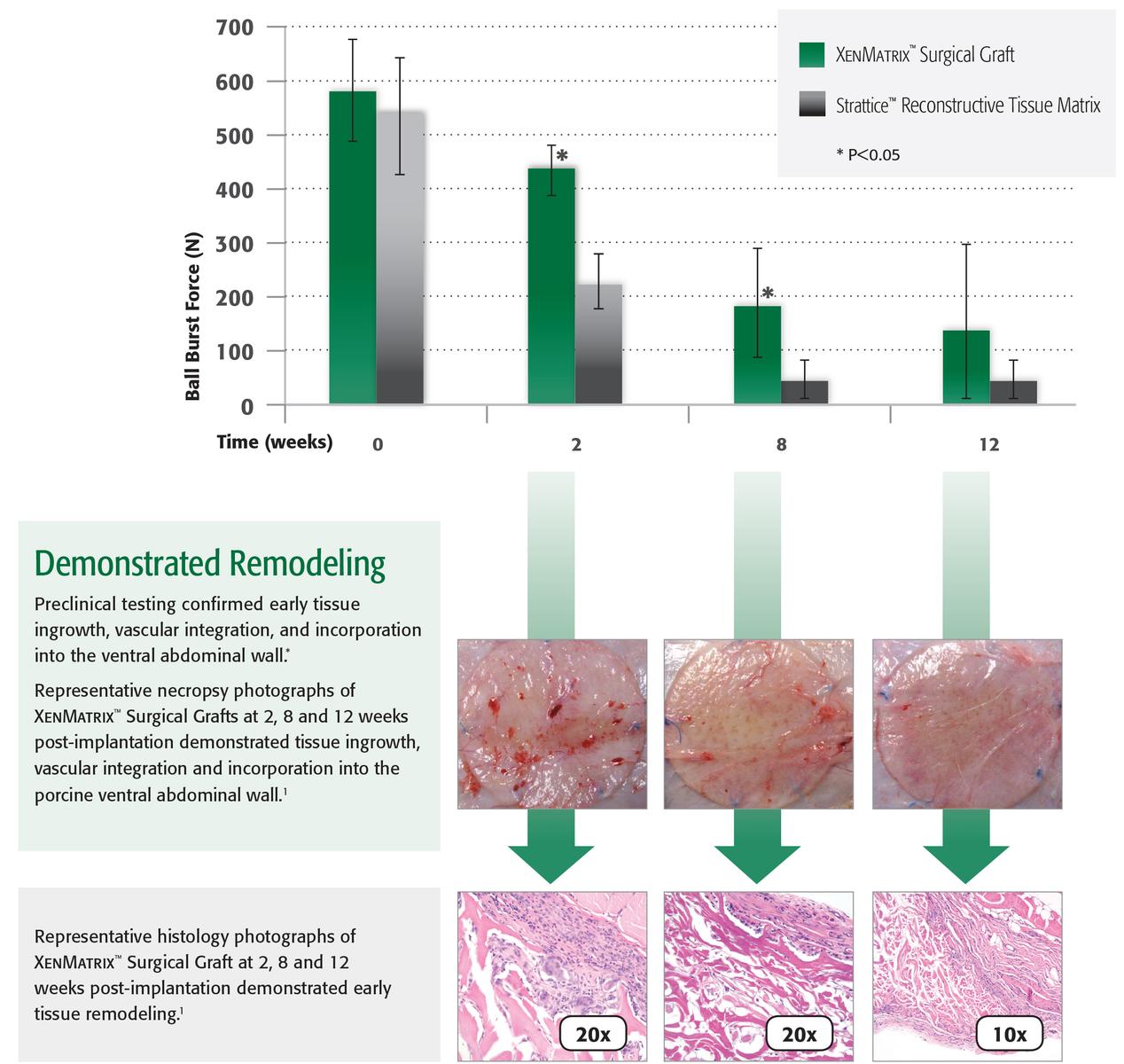
XenMatrix™ Grafts are created using the patented AquaPure™ Process which effectively removes cells while maintaining the structure and strength of the graft. The resulting open collagen scaffold allows early cellular infiltration and revascularization without a significant loss of strength during the early healing process as demonstrated in preclinical studies.1