RELATED PRODUCTS NOT AVAILABLE
true
Support
Sales
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Customer Care
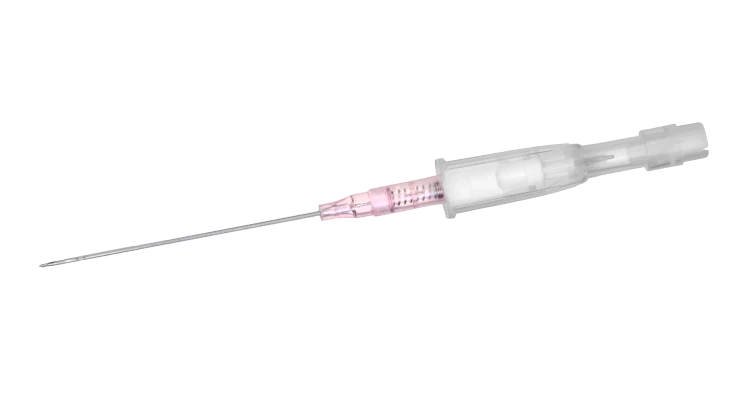
BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology
Connect to confidence, care and comfort.


- Overview
- Products & Accessories
- EIFU & Resources
- FAQ
The BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology has been specially designed to deliver confidence, care and comfort for both the healthcare provider and the patient during everyday procedures.
The ergonomic design of the catheter with discreet curves and an anti-rotation tab provides enhanced control throughout the procedure. The BD Multiguard™ Technology allows a safe insertion reducing the risk of blood exposure to the clinician, whilst a passive safety system provides protection from accidental needlestick injury and associated risk.†
BD Instaflash™ Needle Technology
Designed to confirm immediate visual vessel entry, increasing opportunities for first attempt insertion success.
BD Vialon™ Catheter Material
Softens in the vein up to 70%, enabling longer dwell times,¹* minimizing re-starts and reducing the risk of mechanical phlebitis.1*
BD Multiguard™ Technology
Helps control blood flow at insertion, connections and disconnections,²†† eliminating the need for venous compression and reducing the risk of blood exposure.†
Passive safety
Automatically shields the needle as it is withdrawn, providing protection from needlestick injury and blood exposure
Enhanced confidence
- Reduces blood exposure risk†† by controlling the blood flow during connection / disconnection procedures.††
- Eliminates the need for venous compression during insertion,2† repeated connections and blood draws.
- Provides immediate visual confirmation of vessel entry with BD Instaflash™ Needle Technology, improving opportunities for first attempt insertion success.
- Passive safety system provides protection from needlestick injuries (NSIs).
- Ergonomic design is easily adaptable to preferred insertion techniques and provides enhanced control throughout the procedure.
Improved care
- Decreases the risk of mechanical phlebitis by up to 50% with BD Vialon™ Catheter Material.1*
Increased comfort
- Supports longer dwell times,* minimizing restarts and reinsertions with BD Vialon™ Catheter Material.1
- Design minimizes insertion discomfort with smooth, tapered catheter tip.*
BD Cathena™ Insertion Animation
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes:
BD Cathena™ Safety IV Catheters are suitable for use with power injectors set to a maximum pressure of 325 psi.
†Compared to a non-safety, non-blood control catheter. ††For up to 10 seconds. *Compared to an FEP catheter.
References
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Annals of Internal Medicine. 1991;114:845-854.
- Onia R, Eshu-Wilson I, Arce, C, et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. CMRO. 2011;27(7):1339-1346.
BD-39783 (08/2021)
true
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes:
BD Cathena™ Safety IV Catheters are suitable for use with power injectors set to a maximum pressure of 325 psi.
†Compared to a non-safety, non-blood control catheter. ††For up to 10 seconds. *Compared to an FEP catheter.
References
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Annals of Internal Medicine. 1991;114:845-854.
- Onia R, Eshu-Wilson I, Arce, C, et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. CMRO. 2011;27(7):1339-1346.
BD-39783 (08/2021)
false
true