- Overview
- EIFU & Resources
- No mixing and no refrigeration
- Ready on demand and 5 year shelf life
- Pop the cap and apply powder directly to the bleeding site2
- Thrombin free, biocompatible and non-pyrogenic
- Typically absorbed in 24-48 hours* by amylases
* Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
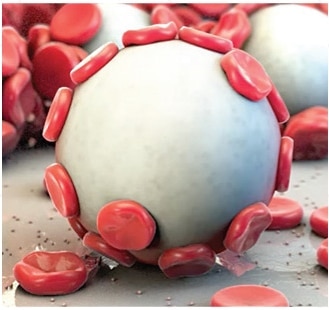
Proprietary MPH™ Technology: A Unique Approach to Achieving Hemostasis
The power of Arista™ AH lies in its proprietary MPH™ (Microporous Polysaccharide Hemospheres) technology. Consisting of microporous particles with a controlled pore size, the spheres are designed to act as a molecular sieve. The powerful osmotic action dehydrates and gels the blood on contact to accelerate the natural clotting process.
- Clotting process begins on contact, regardless of patient’s coagulation status3
- Complete Hemostasis is achieved in minutes1
- Provides broad area coverage on rough surfaces and in hard-to-reach areas
Arista™ AH MOA Animation video
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Arista™ AH PMA P050038 Clinical Study.
See full Instructions for Use for detailed application instructions.
Arista™ AH Instructions for Use.
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
BD-51218 (08/22)