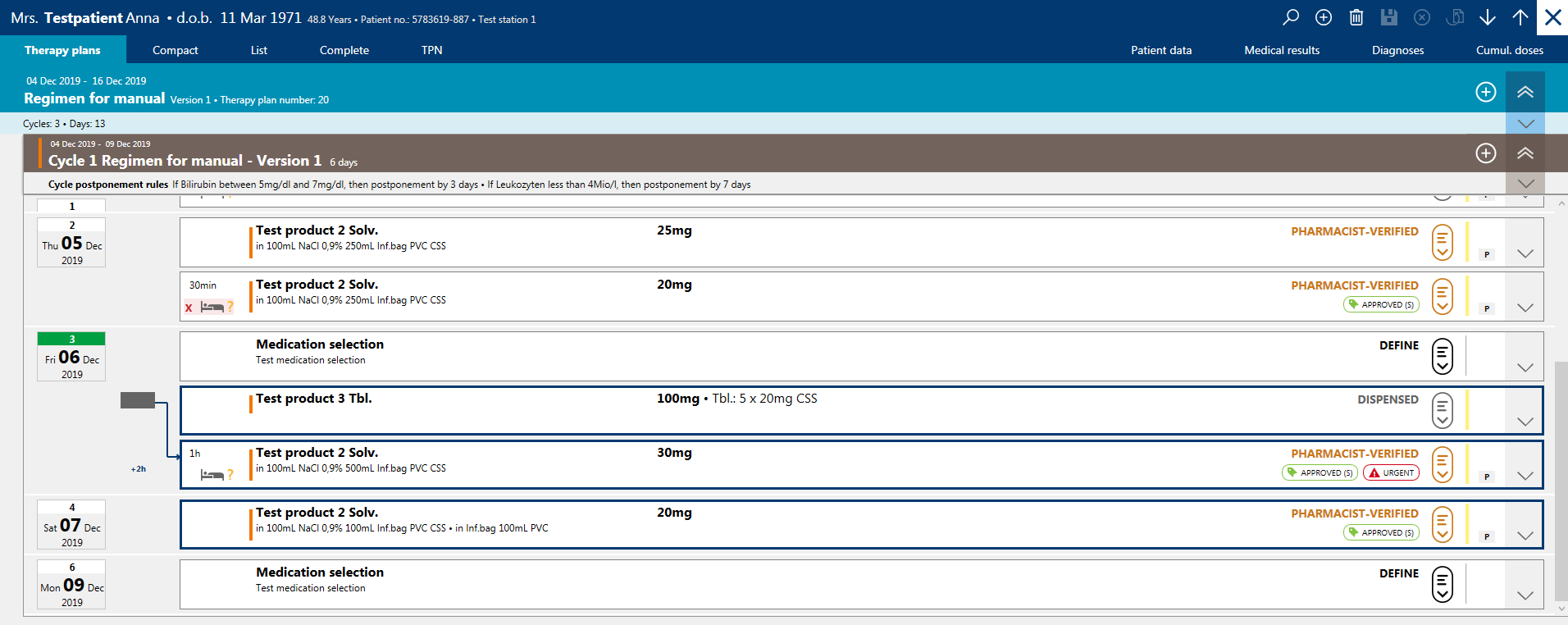
Bed planning solution enables scheduling of chairs and beds available for out-patients. Chairs or beds can be reserved for the duration of an administration and can be assigned to a patient. Any changes in appointments in this module are automatically transmitted to the BD Cato™ Prescribe and BD Cato™ Pharmacy modules.
BD Cato™ has enabled us to improve the quality of our chemotherapy and biologic therapy by stratifying workflow, improving documentation of ordering medication by the physicians as well as preparation of medication in the pharmacy. We have improved co-operation with nurses and physicians in order to decrease medication errors and cut down on labor costs.”
“By having BD Cato™ we have standardised prescription process and reduced the possibilty of errors, caused by transcription, mishandling of paperwork, wrong calculated doses and medications which have been wrongly prescribed. A huge improvement overall offering my team more confidence in their day to day work.”