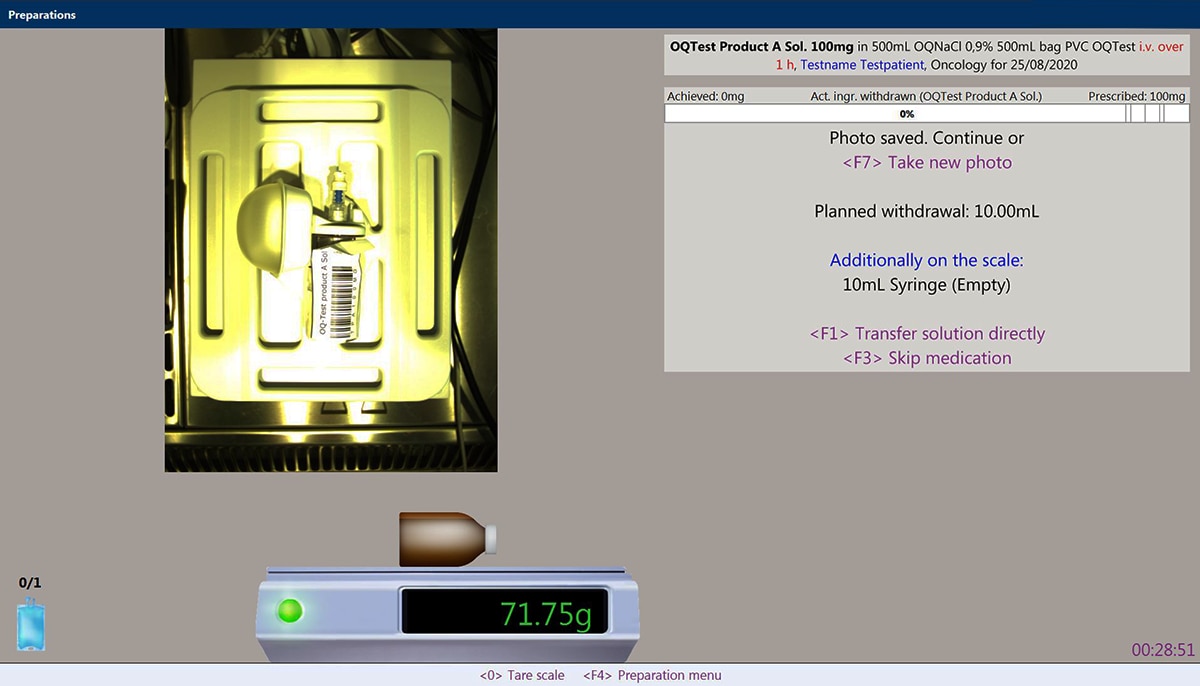
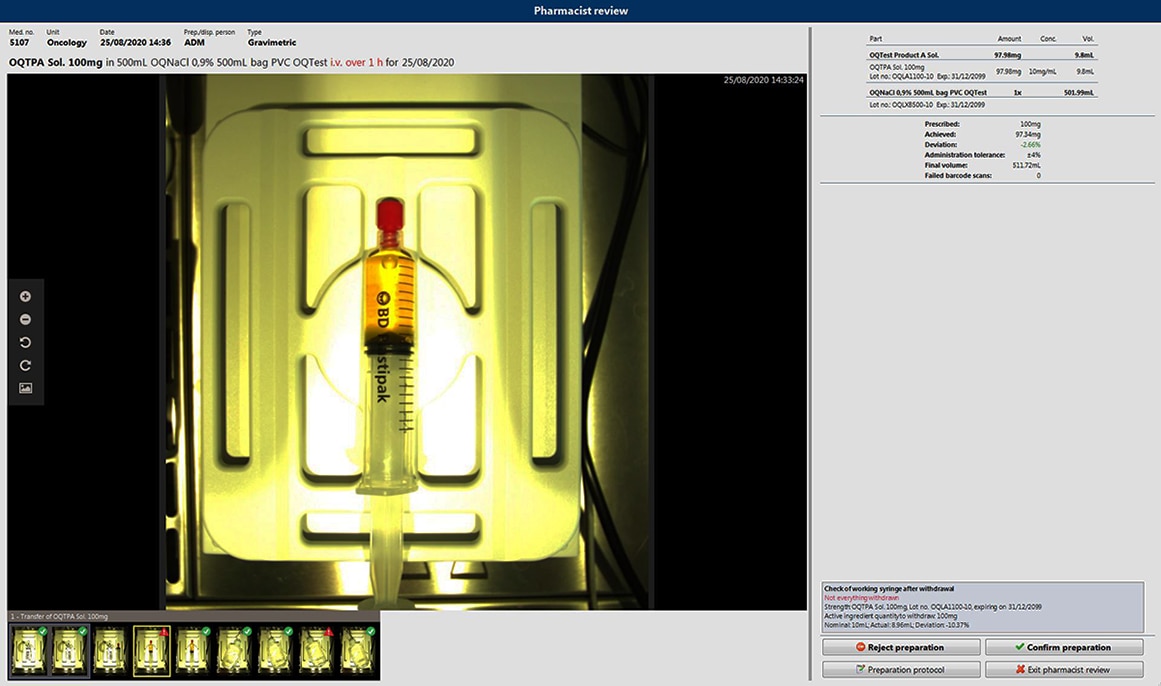
La preparazione di farmaci a somministrazione endovenosa (ad es., farmaci citotossici) conforme alle norme GAMP5 è un processo complesso per tutte le persone coinvolte. C'è la necessità di una documentazione accurata per ogni fase, dall'ordine alla produzione. Questo processo può essere impegnativo e la farmacia deve garantire che la preparazione segua un percorso chiaro e convalidato. Ogni evento imprevisto deve essere gestito tramite le procedure operative standard (SOP), che devono essere rispettate dal personale.
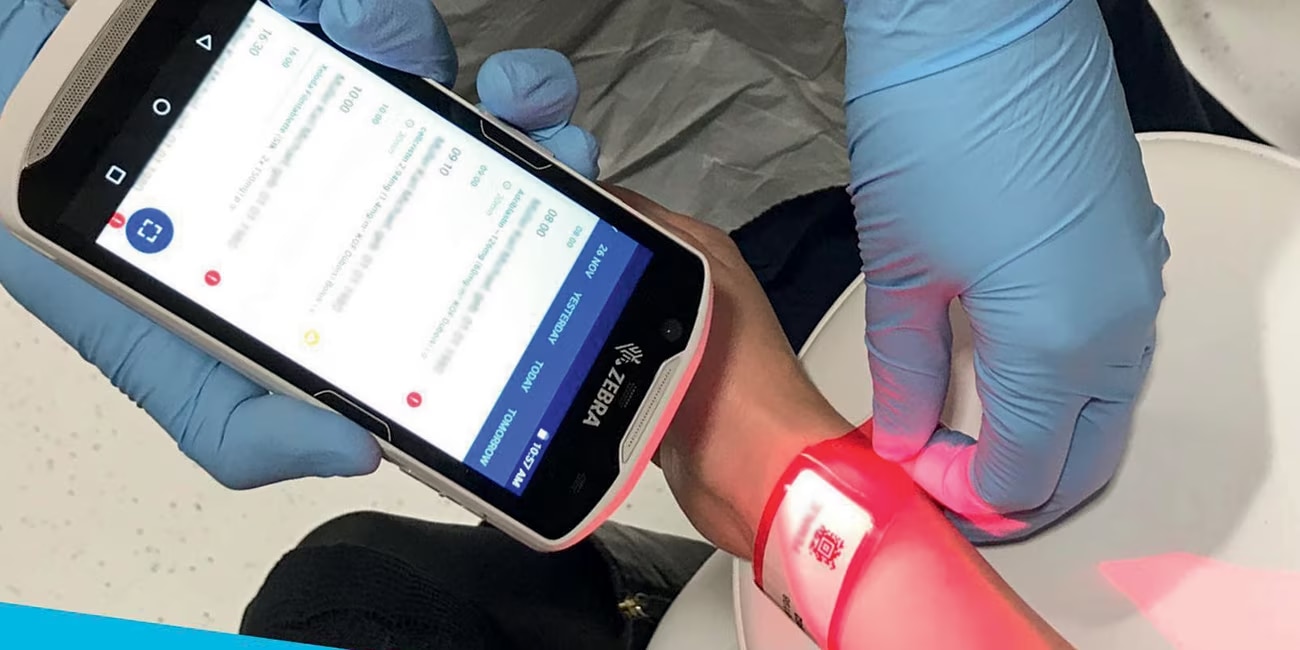
"The Pharmacy department of the Radboud university medical center in the Netherlands has a compounding unit that compounds (preparation for administration) over 60,000 units annually, using the BD Cato™ workflow system. About half of the preparations are products for outpatient care, mainly elastomeric pumps with antibiotics. As the manual filling of large numbers of elastomeric pumps puts a lot of physical stress on the technicians, we have implemented the KIRO® Oncology robot for automated compounding of non-cytostatics, being antibiotics. The BD Cato™ system has been integrated for bi-directional communication with the KIRO® robot and its workflow system KIRO® Link. The two systems have different set-ups and both BD Cato™ as well as Kiro-Grifols have put large effort in incorporating the IV robot into the Radboudumc workflow.
Currently, with the help of BD Cato™ we have bi-directional communication between the Epic hospital system used to prescribe cytostatics and BD Cato™. By putting all the pieces together BD Cato™ enables us to benefit the most from automation to support our workflow for compounding.”