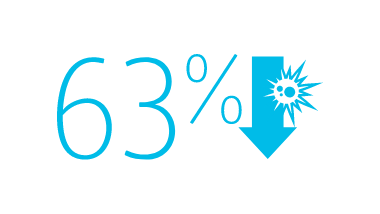
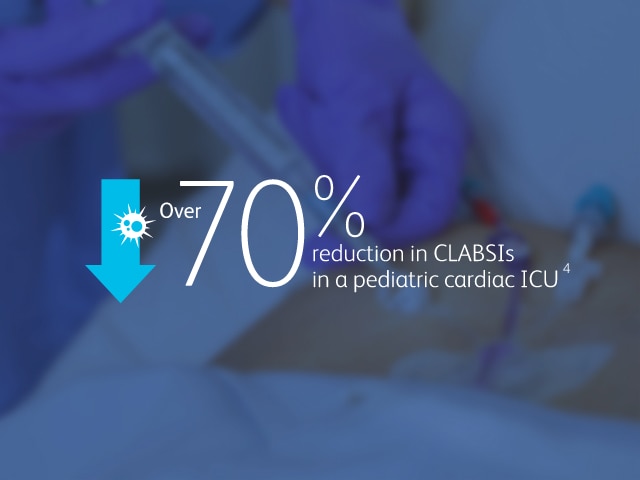
2013 CMS Hospital Compare data reported by 3,075 U.S. hospitals, accounting for nearly 11,000 CLABSIs associated with nearly 10 million catheter days, show that hospitals using the MaxPlus™ Needle-free Connector had lower unadjusted CLABSI rates, as well as lower standardised infection ratios, compared to hospitals not using the MaxPlus™ Needle-free Connector.1,3