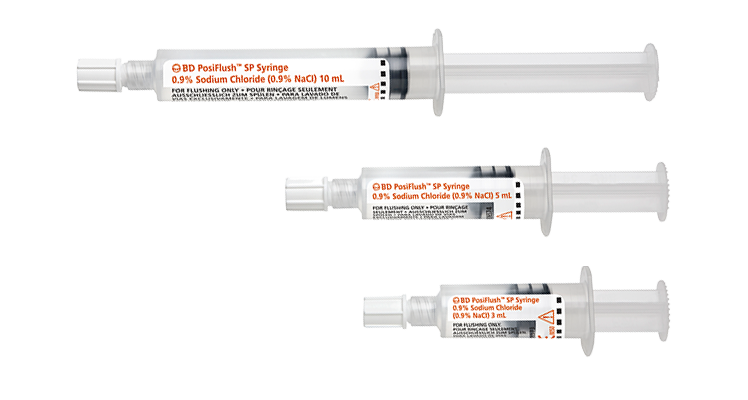
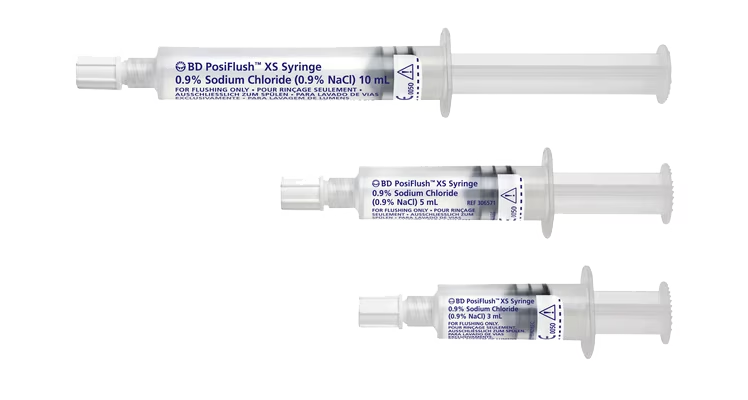
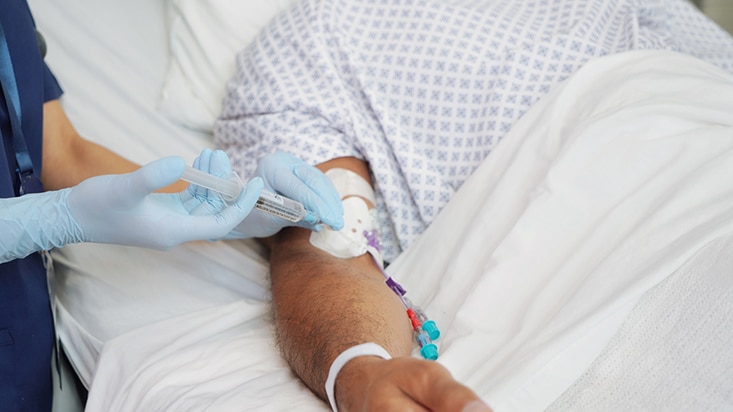
While flushing may seem like just another task, it’s an important part of maintaining patency of the line and reducing the risk of complications, including occlusion, or premature catheter failure. In addition, flushing before and after medication is a strongly recommended step in medication delivery, helping prevent contact between incompatible medicines and ensure that the entire dose of medication is delivered.