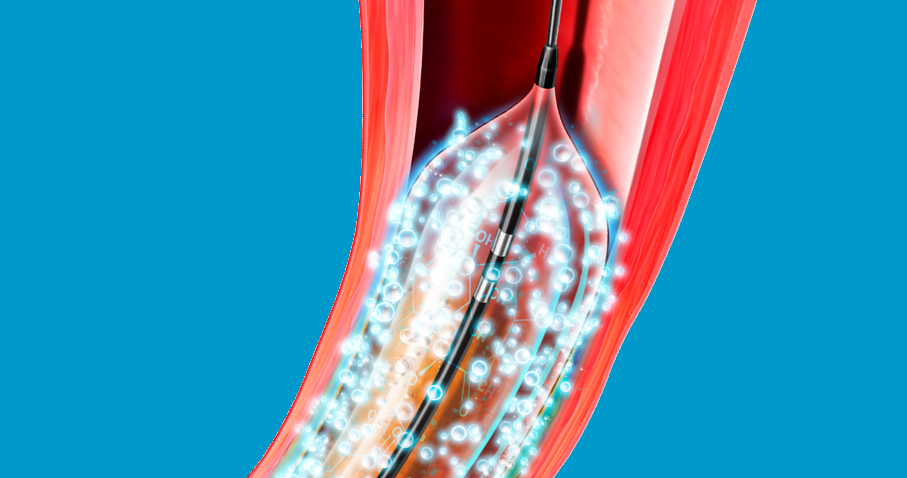
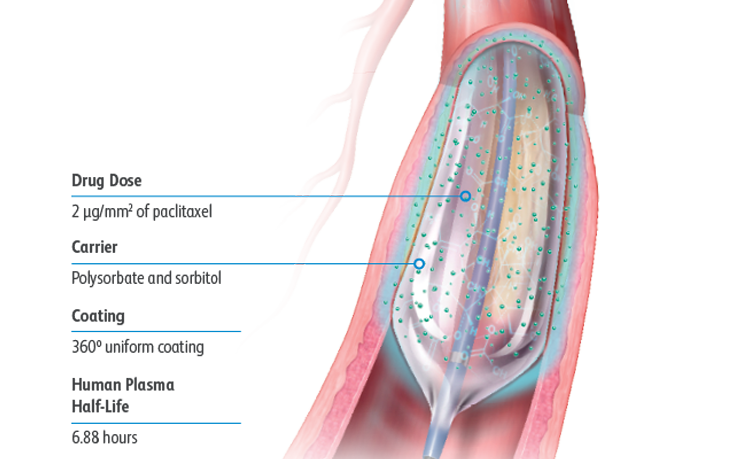
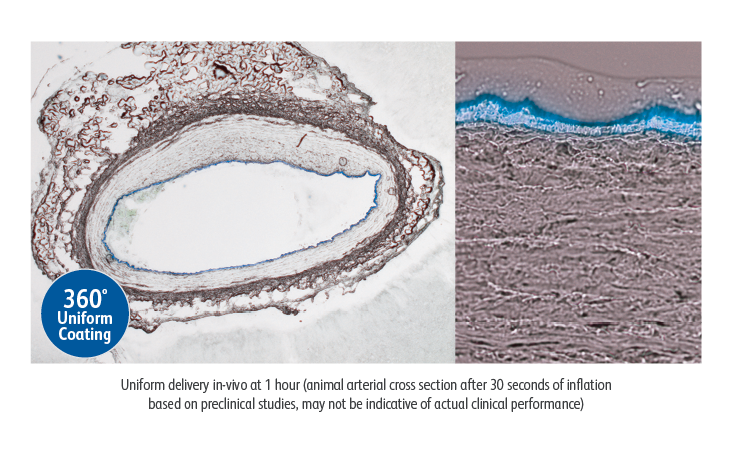
Lutonix™ 018 DCB
Indication for Use
The LUTONIX® 018 Drug Coated Balloon Catheter is intended for Percutaneous Transluminal Angioplasty (PTA), in the femoropopliteal artery and for the treatment of obstructive lesions and decreasing the incidence of restenosis.
In additon, the LUTONIX® 018 Drug Coated Balloon Catheter is intended for PTA of native dialysis fistulae or synthetic grafts to improve blood flow and decrease the incidence of restenosis.
The LUTONIX® 018 Drug Coated Balloon Catheter is intended for treatment up to 290mm in lesions length or approximately a total drug coating quantity of 7.6 mg paclitaxel per patient (see table 1 for drug dosage per each balloon size).
Contraindications
The Lutonix™ DCB Catheter is contraindicated for use in:
1) Patients who cannot receive recommended anti-platelet and/or anticoagulant therapy.
2) Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
3) Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system.
Warnings
1) Contents supplied STERILE using ethylene oxide (EO) process. Do not use if sterile barrier is damaged or opened prior to intended use.
2) Do not use after ‘Use by’ date.
3) Do not use if product damage is evident.
4) The Lutonix™ DCB Catheter is for use in one patient only; do not reuse in another patient, reprocess or resterilise. Risks of reuse in another patient, reprocessing, or resterilisation include: – Compromising the structural integrity of the device and/or device failure which, in turn, may result in patient injury, illness or death. – Creating a risk of device contamination and/or patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to patient injury, illness or death.
5) Do not exceed the Rated Burst Pressure (RBP) recommended for this device. Balloon rupture may occur if the RBP rating is exceeded. To prevent over-pressurization, use of a pressure monitoring device is recommended.
6) Use the recommended balloon inflation medium of contrast and sterile saline (=50% contrast). Never use air or any gaseous medium to inflate the balloon as this may cause air emboli in case of balloon burst.
7) This product should not be used in patients with known hypersensitivity to paclitaxel or structurally related compounds, as this may cause allergic reaction difficulty in breathing, skin rash, muscle pain.
Precautions
1) The Lutonix™ DCB Catheter should only be used by physicians trained in percutaneous interventional procedures.
2) Consideration should be given to the risks and benefits of use in patients with a history of non-controllable allergies to contrast agents.
3) The safety and effectiveness of the Lutonix™ DCB Catheter have not been established for treatment in cerebral, carotid, coronary, or renal vasculature.
4) For SFA application, the safety and effectiveness of using more than four Lutonix drug coated balloons or a maximum drug coating quantity of approximately 15.1 mg paclitaxel in a patient has not been clinically evaluated.
5) For AV Fistula application, the safety and effectiveness of using multiple Lutonix drug coated balloons that deliver greater than 7.6 mg paclitaxel in a patient has not been clinically evaluated.
Use in Conjunction with Other Procedures
The safety and effectiveness of the Lutonix™ DCB Catheter used in conjunction with drug eluting stents or other drug coated balloons in the same procedure or following treatment failure has not been evaluated.
Note: Use with stent graft/bare metal stents for bailout if needed in the same procedure following treatment with the Lutonix™ DCB Catheter is permitted.
Potential Adverse Events
Potential adverse events which may be associated with a peripheral balloon dilatation procedure include, but are not limited to the following: Additional intervention · Allergic reaction to drugs, excipients, or contrast medium · Amputation/loss of limb (SFA) · Aneurysm or pseudoaneurysm · Arrhythmias · Embolization · Hematoma · Hemorrhage, including bleeding at the puncture site · Hypotension/hypertension · Inflammation · Loss of permanent access (AVF) · Occlusion · Pain or tenderness · Pneumothorax or hemothorax (SFA) · Sepsis/infection · Shock · Steal Syndrome (AVF) · Stroke · Thrombosis · Vessel dissection, perforation, rupture, or spasm · Although systemic effects are not anticipated, refer to the Physicians’ Desk Reference for more information on the potential adverse events observed with paclitaxel. Potential adverse events, not described in the above source, which may be unique to the paclitaxel drug coating include: Allergic/immunologic reaction to the drug coating (paclitaxel) · Alopecia · Anemia · Blood product transfusion · Gastrointestinal symptoms · Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia) · Hepatic enzyme changes · Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis · Myalgia/Arthralgia · Myelosuppression · Peripheral neuropathy
Lutonix™ 035 DCB
Indication for Use
The LUTONIX® 035 Drug Coated Balloon Catheter is intended for Percutaneous Transluminal Angioplasty (PTA), in the peripheral vasculature and for the treatment of obstructive lesions and decreasing the incidence of restenosis.
In additon, the LUTONIX® 035 Drug Coated Balloon Catheter is intended for PTA of native dialysis fistulae or synthetic grafts, opening narrowing and immature fistulae, to improve blood flow and decrease the incidence of restenosis.
Contraindications
The Lutonix™ DCB Catheter is contraindicated for use in:
1) Patients who cannot receive recommended anti-platelet and/or anticoagulant therapy.
2) Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
3) Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system.
Warnings
1) Contents supplied STERILE using ethylene oxide (EO) process. Do not use if sterile barrier is damaged or opened prior to intended use.
2) Do not use if product damage is evident.
3) The Lutonix™ DCB Catheter is for use in one patient only; do not reuse in another patient, reprocess or resterilize. Risks of reuse in another patient, reprocessing, or resterilization include: – Compromising the structural integrity of the device and/or device failure which, in turn, may result in patient injury, illness or death. – Creating a risk of device contamination and/or patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to patient injury, illness or death.
4) Do not exceed the Rated Burst Pressure (RBP) recommended for this device. Balloon rupture may occur if the RBP rating is exceeded. To prevent over-pressurization, use of a pressure monitoring device is recommended.
5) Use the recommended balloon inflation medium of contrast and sterile saline (≤50% contrast). Never use air or any gaseous medium to inflate the balloon.
6) This product should not be used in patients with known hypersensitivity to paclitaxel or structurally related compounds.
7) The safety and effectiveness of the Lutonix™ Catheter have not been established for treatment in cerebral, carotid, coronary, or renal vasculature.
8) The safety and effectiveness of using more than four Lutonix™ drug coated balloons or a maximum drug coating quantity of approximately 15.1 mg paclitaxel in a patient has not been clinically evaluated.
Precautions
1) The Lutonix™ DCB Catheter should only be used by physicians trained in percutaneous interventional procedures.
2) Consideration should be given to the risks and benefits of use in patients with a history of non-controllable allergies to contrast agents.
Use in Conjunction with Other Procedures
The safety and effectiveness of the Lutonix™ Catheter used in conjunction with other drug eluting stents or drug coated balloons in the same procedure or following treatment failure has not been evaluated.
Note: Use with bare metal stents for bailout if needed in the same procedure following treatment with the Lutonix™ Catheter is permitted.
Potential Adverse Events
Potential adverse events which may be associated with a peripheral balloon dilatation procedure include: Additional intervention ∙ Allergic reaction to drugs, excipients, or contrast medium ∙ Amputation/loss of limb ∙ Aneurysm or pseudoaneurysm ∙ Arrhythmias ∙ Embolization ∙ Hematoma ∙ Hemorrhage, including bleeding at the puncture site ∙ Hypotension/hypertension ∙ Inflammation ∙ Occlusion ∙ Pain or tenderness ∙ Pneumothorax or hemothorax ∙ Sepsis/infection ∙ Shock ∙ Stroke ∙ Thrombosis ∙ Vessel dissection, perforation, rupture, or spasm ∙ Although systemic effects are not anticipated, refer to the Physicians’ Desk Reference for more information on the potential adverse events observed with paclitaxel. Potential adverse events, not described in the above source, which may be unique to the paclitaxel drug coating include: Allergic/immunologic reaction to the drug coating (paclitaxel) ∙ Alopecia ∙ Anemia ∙ Blood product transfusion ∙ Gastrointestinal symptoms ∙ Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia) ∙ Hepatic enzyme changes ∙ Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis ∙ Myalgia/Arthralgia ∙ Myelosuppression ∙ Peripheral neuropathy
Lutonix™ 014 DCB
Indication for Use
The LUTONIX® 014 Drug Coated Balloon Catheter is intended for use as a PTA catheter to dilate stenotic or obstructive vascular lesions in the lower extremities, for the purpose of improving limb perfusion and decreasing the incidence of restenosis.
Contraindications
The Lutonix™ DCB Catheter is contraindicated for use in:
1) Patients who cannot receive recommended anti-platelet and/or anticoagulant therapy.
2) Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
3) Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system.
Warnings
1) Contents supplied STERILE using ethylene oxide (EO) process. Do not use if sterile barrier is damaged or opened prior to intended use.
2) Do not use if product damage is evident.
3) The Lutonix™ DCB Catheter is for use in one patient only; do not reuse in another patient, reprocess or resterilize. Risks of reuse in another patient, reprocessing, or resterilization include: – Compromising the structural integrity of the device and/or device failure which, in turn, may result in patient injury, illness or death. – Creating a risk of device contamination and/or patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to patient injury, illness or death.
4) Do not exceed the Rated Burst Pressure (RBP) recommended for this device. Balloon rupture may occur if the RBP rating is exceeded. To prevent over-pressurization, use of a pressure monitoring device is recommended.
5) Use the recommended balloon inflation medium of contrast and sterile saline (≤50% contrast). Never use air or any gaseous medium to inflate the balloon.
6) This product should not be used in patients with known hypersensitivity to paclitaxel or structurally related compounds.
7) The safety and effectiveness of the Lutonix™ Catheter have not been established for treatment in cerebral, carotid, coronary, or renal vasculature.
8) The safety and effectiveness of using more than four Lutonix™ drug coated balloons or a maximum drug coating quantity of approximately 15.1 mg paclitaxel in a patient has not been clinically evaluated.
Precautions
1) The Lutonix™ DCB Catheter should only be used by physicians trained in percutaneous interventional procedures.
2) Consideration should be given to the risks and benefits of use in patients with a history of non-controllable allergies to contrast agents.
Use in Conjunction with Other Procedures
The safety and effectiveness of the Lutonix™ Catheter used in conjunction with other drug eluting stents or drug coated balloons in the same procedure or following treatment failure has not been evaluated.
Note: Use with bare metal stents for bailout if needed in the same procedure following treatment with the Lutonix™ Catheter is permitted.
Potential Adverse Events
Potential adverse events which may be associated with a peripheral balloon dilatation procedure include: Additional intervention ∙ Allergic reaction to drugs, excipients, or contrast medium ∙ Amputation/loss of limb ∙ Aneurysm or pseudoaneurysm ∙ Arrhythmias ∙ Embolization ∙ Hematoma ∙ Hemorrhage, including bleeding at the puncture site ∙ Hypotension/hypertension ∙ Inflammation ∙ Occlusion ∙ Pain or tenderness ∙ Pneumothorax or hemothorax ∙ Sepsis/infection ∙ Shock ∙ Stroke ∙ Thrombosis ∙ Vessel dissection, perforation, rupture, or spasm ∙ Although systemic effects are not anticipated, refer to the Physicians’ Desk Reference for more information on the potential adverse events observed with paclitaxel. Potential adverse events, not described in the above source, which may be unique to the paclitaxel drug coating include: Allergic/immunologic reaction to the drug coating (paclitaxel) ∙ Alopecia ∙ Anemia ∙ Blood product transfusion ∙ Gastrointestinal symptoms ∙ Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia) ∙ Hepatic enzyme changes ∙ Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis ∙ Myalgia/Arthralgia ∙ Myelosuppression ∙ Peripheral neuropathy
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions.
BD, the BD logo, and Lutonix are trademarks of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved.
BD-45505v2