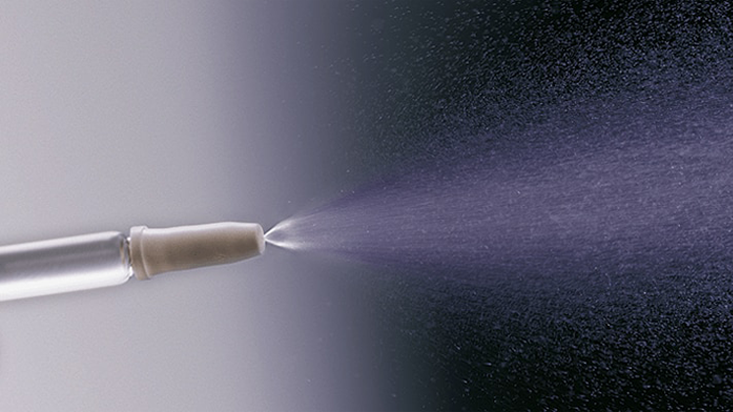
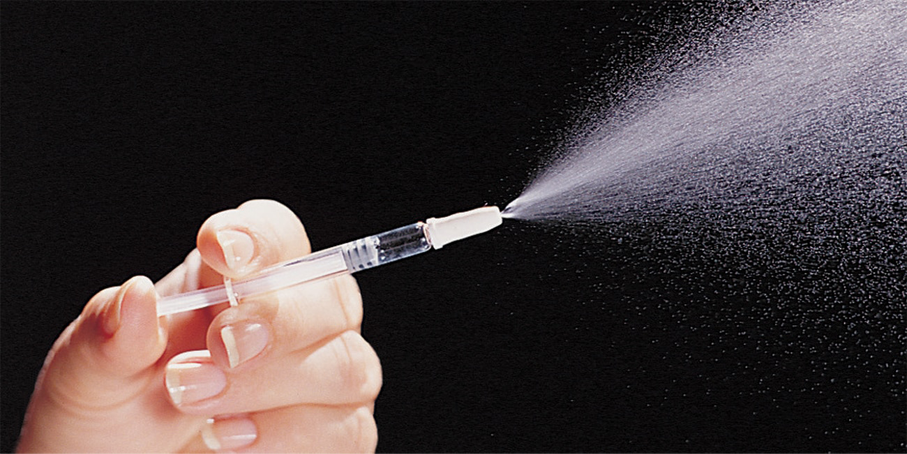
BD Accuspray™ nasal spray system
BD tools for vaccine combination product developers

- Overview
- Expertise & Availability
- Products & Accessories
- Resources
The BD AccusprayTM Nasal Spray System is a monodose or bidose* nasal prefillable delivery system. If given the option between intranasal or injectable vaccination 88.3% adults prefer intranasal delivery.1 Easy to vaccinate2, BD Accuspray™ Nasal Spray System is suitable for high quantities and cold chain when space is critical: small size barrel contributes to reduced storage space. The system is based on BD HypakTM for Vaccines Glass Prefillable Syringe for easy implementation on filling lines.
*if the dose divider is used
BD Accuspray™ Nasal Spray System Features
- Disposable system for nasal administration of vaccines
- Non-reusable, single use nasal sprayer for monodose or bidose* administration
- BD AccusprayTM complies with ISO 10993-13; USP <381>4, <660>5,6, <661>7; Ph Eur 3.1.88, 3.2.16, 3.2.94
*if the dose divider is used
BD Accuspray™ Nasal Spray System Benefits
- Intranasal delivery is preferred 88.3% by adults in the study if given the option between intranasal or injectable vaccination1
- Easy to vaccinate2
- Suitable for high quantities and cold chain9 when space is critical: small size barrel contributes to reduce storage space
- Based on BD HypakTM for Vaccines Glass Prefillable Syringe for easy implementation on filling lines:
o Product can be filled on standard PFS filling line worldwide
o Leverage internal/external filling infrastructure - Products are provided Sterile, Clean and ready to Fill* (BD SCFTM)
- Broad range of value added services*
o Functional tests in c-GMP compliant labs
o Regulatory expertise in Drug Device Combination Product (DDCP) submissions
* Barrel and spray nozzle are delivered assembled, to be further assembled with stopper and plunger rod.
- Sheldon et al. (2013) Immunogenicity of a quadrivalent Ann Arbor strain live attenuated influenza vaccine delivered using a blow-fill-seal device in adults: a randomized, active-controlled study. Influenza and Other Respiratory Viruses 7(6), 1142–1150
- Dubé et al., April 2015, Acceptability of live attenuated influenza vaccine by vaccine providers in Quebec, Canada, Human Vaccines & Immunotherapeutics. Survey conducted to explore knowledge, attitudes and practices of 314 vaccine providers regarding use of LAIV. During the vaccination campaign, 71% of responded having used LAIV delivered with Accuspray. Almost all of these respondents indicated that it was easy to vaccinate children with the vaccine delivered with Accuspray (57% strongly agreed)
- Materials Of Concern And Safety Information, 442.MOCASI.28, valid from April 2021
- USP <381> “Elastomeric Components in Injectable Pharmaceutical Product Packaging/Delivery Systems” (Dec. 2020) and EP 3.2.9 “Rubber Closures for Containers for Aqueous Parental Preparations, for powders and for freeze-dried powders” (Jul 2018) compliance statement for W7028/ 55, STMT-QE20213696, Sept. 2021
- USP <660> “Containers-Glass” (May 2015), STMT-20161598, April 2021
- Hydrolytic resistance conformity of glass canes to the new version of EP 3.2.1.“Glass containers for pharmaceutical use”, STMT-QE20191153, April 2019
- USP <661> “Plastic packaging systems and their materials of construction”, STMT-QE20213531, Sept. 2021
- Ph Eur 3.1.8 "Silicone oil used as a lubricant“, STMT-QE20170709, Dec 2020
- BD internal references, EF20202208, EF20202618, EF20203052,TP20211855, TR20213724, EF20213171 BD-01-SR-01, BD-02-SR-01, BD-03-SR-01, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2021.
Our Expertise in the BD AccusprayTM Nasal Spray System
- More than than 150 million BD AccusprayTM units10 sold since 2003
Availability
- Samples available on demand
- Commercial availability to be evaluated against requirements
- Sheldon et al. (2013) Immunogenicity of a quadrivalent Ann Arbor strain live attenuated influenza vaccine delivered using a blow-fill-seal device in adults: a randomized, active-controlled study. Influenza and Other Respiratory Viruses 7(6), 1142–1150
- Dubé et al., April 2015, Acceptability of live attenuated influenza vaccine by vaccine providers in Quebec, Canada, Human Vaccines & Immunotherapeutics. Survey conducted to explore knowledge, attitudes and practices of 314 vaccine providers regarding use of LAIV. During the vaccination campaign, 71% of responded having used LAIV delivered with Accuspray. Almost all of these respondents indicated that it was easy to vaccinate children with the vaccine delivered with Accuspray (57% strongly agreed)
- Materials Of Concern And Safety Information, 442.MOCASI.28, valid from April 2021
- USP <381> “Elastomeric Components in Injectable Pharmaceutical Product Packaging/Delivery Systems” (Dec. 2020) and EP 3.2.9 “Rubber Closures for Containers for Aqueous Parental Preparations, for powders and for freeze-dried powders” (Jul 2018) compliance statement for W7028/ 55, STMT-QE20213696, Sept. 2021
- USP <660> “Containers-Glass” (May 2015), STMT-20161598, April 2021
- Hydrolytic resistance conformity of glass canes to the new version of EP 3.2.1.“Glass containers for pharmaceutical use”, STMT-QE20191153, April 2019
- USP <661> “Plastic packaging systems and their materials of construction”, STMT-QE20213531, Sept. 2021
- Ph Eur 3.1.8 "Silicone oil used as a lubricant“, STMT-QE20170709, Dec 2020
- BD internal references, EF20202208, EF20202618, EF20203052,TP20211855, TR20213724, EF20213171 BD-01-SR-01, BD-02-SR-01, BD-03-SR-01, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2021.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.