Ready to learn more? Let’s have a conversation.
- Overview
- Recognizing the Risks
- Safe Handling Assessment
- Guideline Support & Education
- Related Products
Solutions you can count on
You shouldn’t have to compromise on hazardous drug safety.
From the compounding pharmacist to the nurse who administers treatment to the staff cleaning a facility, an exposure can occur at almost any moment during the hazardous drug journey. The risk of hazardous drug exposure to healthcare workers is real and significant.
There’s no room for compromise.
With evidence-based insights on your current safe handling practices, rapid hazardous drug detection and closed-system drug transfer device (CSTD) solutions—all designed to support alignment with industry guidelines—the BD® Hazardous Drug Solutions portfolio is designed to help protect you—and the team you count on.
Assess. Plan. Protect
The BD® Hazardous Drug Solutions team collaborates with you to understand the real risks of hazardous drug exposure and surface contamination in your facility—and then tailor the solution that’s right for your safety, economic and clinical needs.
Protecting your healthcare workers starts with understanding your facility’s risk. The BD® Safe Handling Assessment provides a data-driven evaluation of potential hazardous drug exposure, identifying potential gaps in compliance, processes and training.
Using proprietary digital tools and industry benchmarks, we deliver an actionable roadmap with BD product recommendations and training to support your efforts to reduce exposure and improve safety.
Focused on surface contamination risks, this unique end-to-end program is designed to help you understand, develop and implement hazardous drug surface contamination monitoring in four steps: assess, develop, test, track.
BD® HD Check System
The first rapid hazardous drug detection* system that provides easy-to-read binary results in less than 10 minutes to help facilitate routine monitoring and evaluate your institution’s safe handling practices.
*The BD® HD Check System tests for select hazardous drugs— cyclophosphamide, doxorubicin and methotrexate. Surfaces with contamination at or above the limits of detection have 95% specificity and sensitivity.
BD® PhaSeal™ Optima System
The PhaSeal™ System pioneered the category of CSTDs to help protect the healthcare workers who prepare and administer hazardous drugs. Twenty years later, we turned to healthcare professionals like you for feedback and guidance. The result is the PhaSeal™ Optima System—a next-generation, airtight and leakproof CSTD solution designed to maximize drug extraction*† while advancing safety.
*Compared to similar systems
†Bench test results may not necessarily be indicative of clinical performance
BD® Texium™ System
The Texium™ System utilizes mechanical valve technology to achieve a leak-free drug transfer and promote the safe handling of hazardous drugs—providing pharmacists and nurses with a simple and cost-effective solution.
Solutions with demonstrated impact
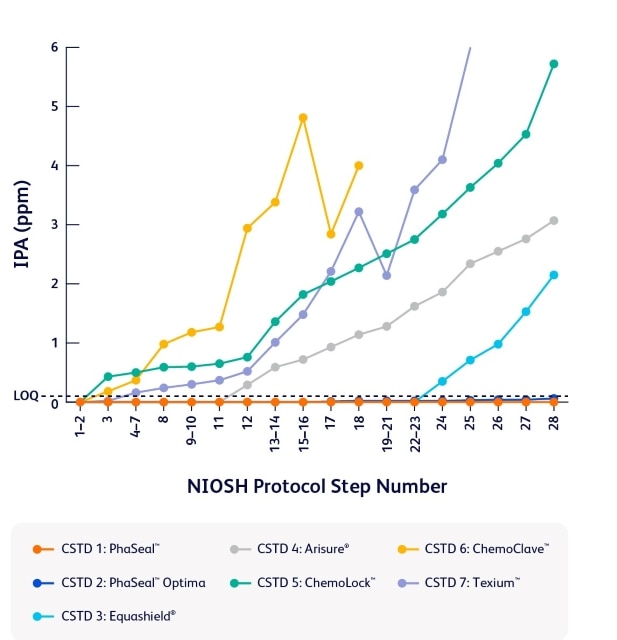
A study published in the Journal of Oncology Pharmacy Practice revealed that of seven tested, the PhaSeal™ Family of CSTDs performed the best in the study to contain vapors during preparation and administration tasks—delivering reliable performance when it matters most.1
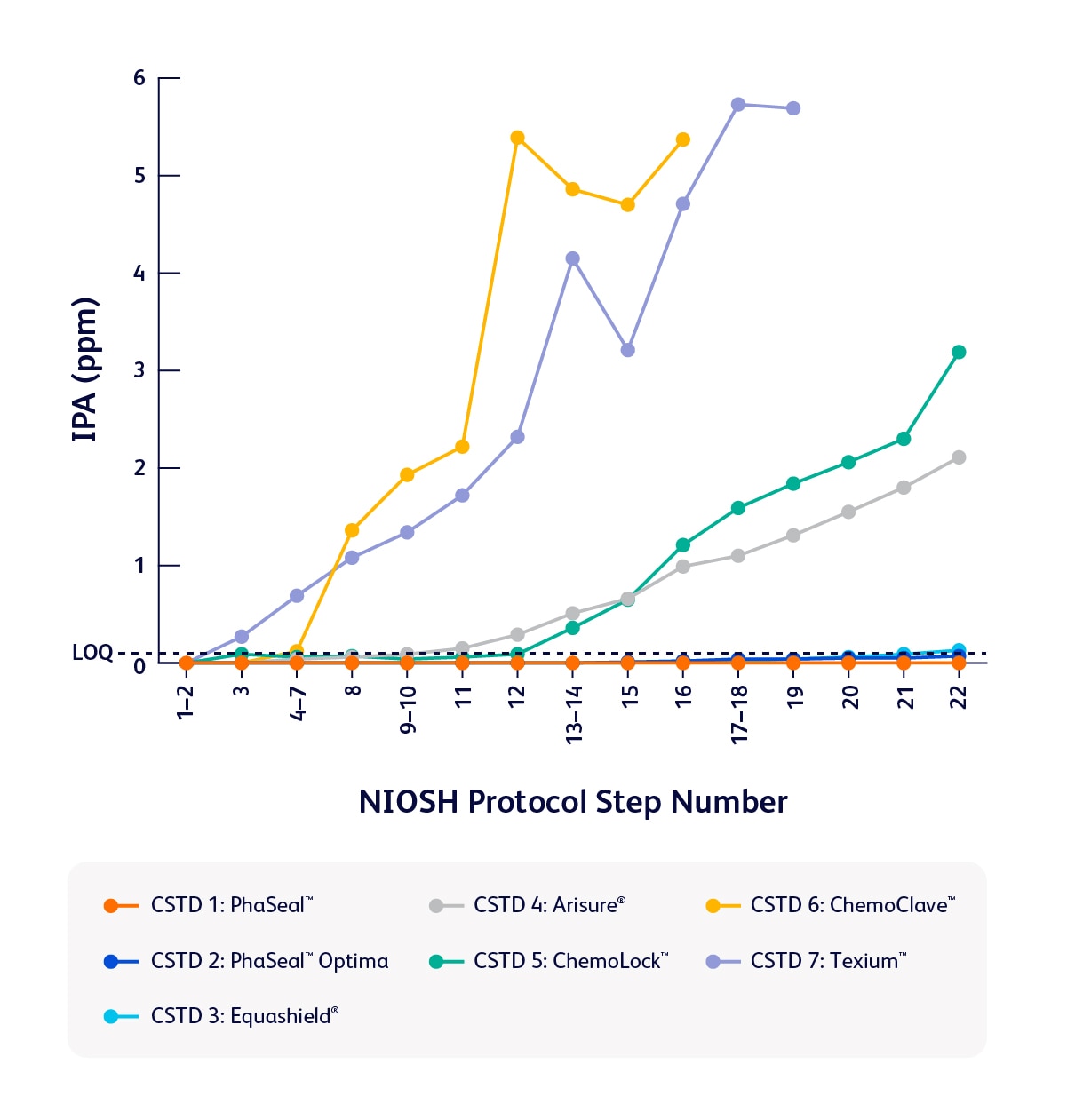
Comparison of task 1 results
A study published in the American Journal of Health-System Pharmacy (AJHP) highlighted the value of integrating PhaSeal™ Optima System and BD® HD Check System into routine clinical practice.2
-

46% reduction in hazardous drug contamination after incorporating BD PhaSeal™ Optima System into clinical workflows1
-

Of the 5 hazardous drugs that showed pretest contamination at one testing site, 4 showed fewer contaminated surfaces post-test, and ALL showed reduced post-test contamination concentrations1
-

As part of a multifaceted approach, the PhaSeal™ Optima System and BD HD Check System may help minimize barriers to routine monitoring, ultimately improving the safety of healthcare workers and patients2
Ready to advance your facility’s safe handling practices? Let’s get started.
- Armistead LT, Stepanovic M, Earnhart MA, Eckel SF. An assessment of seven closed system transfer devices in accordance with the 2015 NIOSH vapor containment performance protocol. J Oncol Pharm Pract. Published online December 10, 2024. doi:10.1177/10781552241304638.
- Brechtelsbauer E. Identification and reduction of hazardous drug surface contamination through the use of a novel closed-system transfer device coupled with a point-of-care hazardous drug detection system. Am J Health Syst Pharm. 2023;80(7):435-444. doi:10.1093/ajhp/zxac336.
BD-2062 (07/25)
Widespread,
well-documented risk
Every year, more than 8 million healthcare workers face potential exposure to hazardous drugs.
From pharmacists and nurses to environmental services and shipping staff, the risk extends across the entire medication journey.1 While these drugs are essential for patient care, their presence in the workplace demands a higher standard of safety.
Potential for serious consequences
The risks of hazardous drug exposure are real—and potentially life-altering
Chromosomal aberrations
A 2.5-fold to 5-fold increase in total chromosomal aberrations has been found among healthcare workers handling hazardous drugs2-4

Organ damage
Nurses handling antineoplastic drugs have an increased risk of liver damage7

Cancer risks
A 1.5-fold increase in nonmelanoma skin cancer and 3.7-fold increase in non-Hodgkin lymphoma has been observed among pharmacy techs5
Nurses exposed to hazardous drugs face a 10.65-fold higher risk of leukemia compared to those without exposure6

Reproductive issues
Staff handling antineoplastic drugs face 2x the risk of miscarriage,8 along with an increased risk of birth defects in their children9
Exposure to hazardous drugs may also present a reproductive risk for men and women actively trying to conceive10
Exposure can occur anywhere on the HD journey
The risk of hazardous drug exposure isn’t confined to a single step. Exposure may occur anywhere hazardous drugs are present in your facility—from pharmacy receiving and preparation to administration and disposal.
A study of six hospitals found measurable levels of hazardous drug contamination on frequently contacted surfaces at every stage of the hospital medication system.11
It’s time to assess and advance your facility’s safe handling practices. BD can help.
- Centers for Disease Control and Prevention (CDC). Hazardous drug exposures in health care. The National Institute for Occupational Safety and Health (NIOSH) Web site. http://www.cdc.gov/niosh/topics/hazdrug. Accessed April 11, 2023.
- Cavallo D, Ursini CL, Perniconi B, et al. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat Res. 2005;587(1-2):45–51. doi:10.1016/j.mrgentox.2005.07.008.
- McDiarmid MA, Oliver MS, Roth TS, Rogers B, Escalante C. Chromosome 5 and 7 abnormalities in oncology personnel handling anticancer drugs. J Occup Environ Med. 2010;52(10):1028–34. doi:10.1097/JOM.0b013e3181f73ae6.
- Roussel C, Witt KL, Shaw PB, Connor TH. Meta-analysis of chromosomal aberrations as a biomarker of exposure in healthcare workers occupationally exposed to antineoplastic drugs. Mutat Res. 2019;781:207-217
- Hansen J, Olsen JH. Cancer morbidity among Danish female pharmacy technicians. Scand J Work Environ Health. 1994;20(1):22–26. doi:10.5271/sjweh.1433.
- Skov T, Maarup B, Olsen J, et al. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs. Br J Ind Med. 1992;49(12):855–861. doi:10.1136/oem.49.12.855.
- Sotaniemi EA, Sutinen S, Arranto AJ, et al. Liver damage in nurses handling cytostatic agents. Acta Med Scand. 1983;214(3):181–189. doi:10.1111/j.0954-6820.1983.tb08593.x.
- Lawson CC, Rocheleau CM, Whelan EA, et al. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol. 2012;206(4):327.e1-8.
- Hemminki K, Kyronen P, Lindbohm ML. Spontaneous abortions and malformations in the offspring of nurses exposed to anaesthetic gases, cytostatic drugs, and other potential hazards in hospitals, based on registered information of outcome. J Epidemiol Community Health. 1985;39(2):141–147. doi:10.1136/jech.39.2.141.
- National Institute for Occupational Safety and Health (NIOSH). Current intelligence bulletin 68: NIOSH chemical carcinogen policy. By Whittaker C, Rice F, McKernan L, Dankovic D, Lentz TJ, MacMahon K, Kuempel E, Zumwalde R, Schulte P, on behalf of the NIOSH Carcinogen and RELs Policy Update Committee. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2017‐100. https://www.cdc.gov/niosh/docs/2017‐100/pdf/2017‐100.pdf. Accessed March 20, 2020.
- Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg. 2013;10(7):374–383. doi:10.1080/15459624.2013.789743.
Assessing current practices
Your facility likely already has safety measures that help prevent hazardous drug exposure. How well is your program protecting your staff? Are you up to date with evolving standards and guidelines? Is your staff in compliance? Are there opportunities to optimize your approach?
BD can help uncover the answers.
BD Safe Handling Assessment
Hazardous drug exposure poses serious risks to healthcare workers, yet it can be difficult to detect and prevent. The BD Safe Handling Assessment provides a data-driven, end-to-end evaluation of your facility’s hazardous drug handling practices—from procurement to disposal.
Using a proven methodology and proprietary digital tools, our experts conduct on-site assessments, including interviews, observations and surface contamination testing, to establish a baseline and identify gaps. Results are delivered in a web-based dashboard with industry benchmarks and evidence-based recommendations—giving you a clear, actionable plan to enhance safety, standardize best practices and support compliance with the latest guidelines.
Get a clear picture of your facility’s risks—and a plan to address them. Contact us today to schedule an assessment.
Additional assessment resources

Joint Commission Resources (JCR) Assessment and Toolkit
Developed by the JCR and sponsored by BD, the Self-Assessment of Safe Handling Practices for Hazardous Drugs is a confidential online survey used to identify existing USP <800> compliance gaps. For help on closing those gaps, the Improving Safe Handling Practices for Hazardous Drugs Toolkit provides detailed guidance on standards, guidelines and best practices.

BD HD Surface Contamination Monitoring Program
The BD HD Surface Contamination Monitoring Program is a unique end-to-end program designed to help your facility set up, customize and sustain surface contamination monitoring in just four steps: assess, develop, test and track. The program, along with the BD HD Check System, puts the power of providing data-driven continuous quality improvements for a safe work environment directly in your hands.
Implementing an HD surface contamination monitoring program - Pharmacy Areas
Implementing an HD surface contamination monitoring program - Admin Areas
Ready to take a closer look at your hazardous drug safety practices?
Set up a Safe Handling Assessment today.
Is your facility up to date with current best practices?
For decades, clinical organizations have provided evidence-based recommendations to help protect healthcare workers from the risks of hazardous drug exposure. As protective technology and knowledge advance, these guidelines evolve. Staying current is a critical part of keeping your team safe.
On-demand webinars and resources
Evidence-based webinars from healthcare experts to enhance knowledge and strengthen skills
Closed-system drug-transfer device (CSTD) usage guidelines
The use of CSTDs has been widely recommended by different organizations, including the United States Pharmacopeial Convention (USP),1 American Society of Health-System Pharmacists (ASHP),2 National Institute for Occupational Safety and Health (NIOSH)3 and Oncology Nursing Society.4
“CSTDs should be used when compounding HDs when dosage form allows.”
“CSTDs must be used for administration of antineoplastic HDs when the dosage form allows.”
“Use and maintenance of proper engineering controls (e.g., C-PECs, C-SECs, and CSTDs).”
“HDs must be administered safely using protective medical devices and techniques, examples of protective medical devices include needleless and closed systems.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Consider using devices such as closed-system transfer devices, glove bags, and needleless systems when transferring hazardous drugs from primary packaging (such as vials) to dosing equipment (such as infusion bags, bottles, or pumps).”
“Develop workplace procedures for using and maintaining all equipment that functions to reduce exposure—such as ventilated cabinets, closed-system drug-transfer devices, needle-less systems, and PPE.”
— NIOSH alert 2004: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings.3
“For these reasons, USP chapter 800 has determined that CSTDs should be used when compounding HDs and that CSTDs must be used when administering antineoplastic HDs when the dosage form allows and the device is physically or chemically compatible with the HD to be used.”
— ASHP Guidelines on Handling Hazardous Drugs2
Surface contamination monitoring guidelines
Organizations, health agencies and conferences around the world, such as the USP1 and ASHP2, recommend routine monitoring as part of a comprehensive safe handling program.
“Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every six months, or more often as needed, to verify containment). […] Repeat the wipe sampling to validate that the deactivation/decontamination and cleaning steps have been effective.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Surface wipe sampling of healthcare settings for HD contamination is advocated as a means of environmental quality and control. Surface wipe sampling should be done routinely, first to determine a benchmark of contamination and then to monitor the effectiveness of safe handling programs.”
— ASHP Guidelines on Handling Hazardous Drugs2
“Each element of the monitoring program must be included in a sampling plan with sample locations, methods of collection, sampling frequency, and other specifics depending on the type of monitoring being performed.”
“Records of data collected through the monitoring program must be maintained as part of the overall quality assurance program of the facility.”
“Ensuring a safe compounding environment requires viable and nonviable airborne particle testing, pressure differential or displacement airflow measurement, temperature monitoring, and surface disinfection sampling and assessment. Each element…must be included in a sampling plan with sample locations, methods of collection, sampling frequency, and other specifics depending on the type of monitoring being performed.”
— ASHP Guidelines on Compounding Sterile Preparations5
Recommended sampling locations
Standards and guidelines have identified several locations in pharmacy and patient administration areas where routine wipe sampling for HD surface contamination might be beneficial.
test the section name text
test the section description text

test the section name text
test the section description text

Training and education recommendations
Comprehensive safe handling programs include comprehensive training and routine reassessments. Both the USP1 and Oncology Nursing Society4 provide specific recommendations for annual HD education.
“All personnel who handle HDs must be trained based on job functions… Personal competency must be reassessed every 12 months.”
— USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings1
“Comprehensive didactic education and documentation of clinical competence is required for all HCW and must be reassessed at least every 12 months.”
— Oncology Nursing Society—Safe Handling of Hazardous Drugs4
Visit the BD Learning Academy
Your partner on best practices
Ample evidence exists to support the prevalence of hazardous drug exposure and associated health risks to healthcare workers. Implementing a comprehensive safe handling program can pose multiple challenges. It’s a step worth taking.
From our BD Safe Handling Assessment to tailoring a cost-effective hazardous drug safety solution that’s right for your facility, BD can help.
Ready to find out how up to date your facility is with guidelines and best practices?
Reach out to learn more about a BD Safe Handling Assessment today.
- United States Pharmacopeial Convention. USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare. Published February 1, 2016. Updated June 26, 2020. Accessed April 11, 2023.
- Power LA, Coyne JW. ASHP Guidelines on Handling Hazardous Drugs. Am J Health Syst Pharm. 2018;75(24):1996-2031. doi:10.2146/ajhp180564
- National Institute for Occupational Safety and Health (NIOSH). NIOSH alert 2004: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf. Accessed August April 11, 2023.
- Polovich M, Olsen, M eds. Safe Handling of Hazardous Drugs. 3rd ed. Pittsburgh, PA: Oncology Nursing Society; 2018.
- American Society of Health-System Pharmacists (ASHP). ASHP guidelines on compounding sterile preparations. Am J Health Syst Pharm. 2014;71(2):145-166. Accessed June 10, 2025. https://www.ashp.org/-/media/assets/policy-guidelines/docs/guidelines/compounding-sterile-preparations.ashx
- Valero-García S, González-Haba E, Gorgas-Torner MQ, et al. Monitoring contamination of hazardous drug compounding surfaces at hospital pharmacy departments. A consensus Statement. Practice guidelines of the Spanish Society of Hospital Pharmacists (SEFH). Farm Hosp. 2021;45(2):96–107. doi:10.7399/fh.11655.
- Gabay M, Johnson P, Fanikos J, et al. Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. Am J Health Syst Pharm. 2021;zxab134. doi:10.1093/ajhp/zxab134.
- Domingo T, Fontán G, Enríquez M, et al. Guía monitorización de superficies de medicamentos peligrosos. 1. ed. Instituto Español de Investigación Enfermera; Consejo General de Enfermería; 2021.
Related Products
-

The first rapid hazardous drug detection* system that provides easyto- read binary results in less than 10 minutes to help facilitate routine monitoring and evaluate your institution’s safe handling practices.
*The BD® HD Check System tests for select hazardous drugs— cyclophosphamide, doxorubicin and methotrexate. Surfaces with contamination at or above the limits of detection have 95% specificity and sensitivity. -
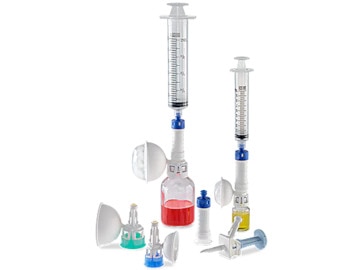
The BD PhaSeal™ System pioneered the category of CSTDs to help protect the healthcare workers who prepare and administer hazardous drugs. Twenty years later, we turned to healthcare professionals like you for feedback and guidance to optimize its every component. The result is the BD PhaSeal™ Optima System—a next-generation, airtight and leakproof CSTD solution designed to maximize drug extraction*† while advancing safety.
*Compared to similar systems
†Bench test results may not necessarily be indicative of clinical performance -
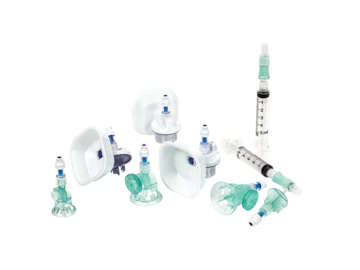
The Texium™ System utilizes mechanical valve technology to achieve a leak-free drug transfer and promote the safe handling of hazardous drugs—providing pharmacists and nurses with a simple and cost-effective solution.
-
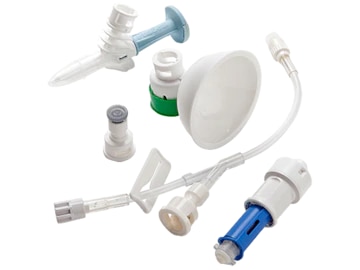
The BD PhaSeal System is airtight and leakproof to protect your staff from hazardous drug exposure during drug preparation, administration and disposal.