Support and strength for the long run1-3
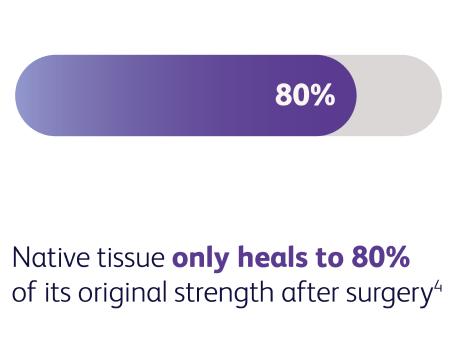
After the age of 20, our skin loses about 1% of its collagen each year.4 This gradual loss of collagen is what causes skin to become thinner and less elastic, resulting in natural sagging and wrinkles.5,6 Many factors can accelerate this effect, including surgery—after which the integrity of the original tissue structures may never be fully restored to their native state.7-10
GalaFLEX™ Scaffold is stronger for longer1,2,12
The biologically derived, P4HB-based GalaFLEX™ Scaffold collection is here to support your plastic and reconstructive surgeries—providing immediate soft tissue reinforcement and a foundation for long-term strength.1,2,13,18,19
-
Biologically Derived
Produced by a standard biological fermentation process13
-
Monofilament
Designed to minimize risk of infection and encourage a natural healing response1,14,15,17
-
Strong
Provides a lattice for new tissue ingrowth, resulting in a tissue plane that is 2-4x stronger than native tissue1,2,12
-
Bioabsorbable
Naturally broken down to CO2 and H2O and bioabsorption is essentially complete by 18-24 months1,2,17,19
-
3-Dimensional
The first and only formed absorbable scaffold (3D and 3D with rim) designed to fit and elevate the body's natural shape, providing easier placement and potentially reduced procedure time1, 20
-
Predictable Performance
Promotes healing and stability due to rapid integration, predictable strength and absorption profile1,2.11.18
-
Lightweight, low-profile options
Also available in a 30% thinner profile, designed to be flexible to provide anatomical compliance1
A strong repair1,2,12
GalaFLEX™ Scaffold has been demonstrated to provide 2-4x greater strength than native tissue at 12-months following implantation1,2,11
Contact us
GalaFLEX™, GalaFLEX 3D™ and GalaFLEX 3DR™ Scaffolds are indicated for use as bioresorbable scaffolds for soft tissue support and to repair, elevate, and reinforce deficiencies where weakness or voids exist that require the addition of material to obtain the desired surgical outcome. This includes reinforcement of soft tissue in plastic and reconstructive surgery, and general soft tissue reconstruction. These products, referred to as the GalaFLEX™ Scaffold collection, are also indicated for the repair of fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
GalaFLEX LITE™ Scaffold is intended to reinforce soft tissue where weakness exists in patients undergoing plastic and reconstructive surgery, or for use in procedures involving soft tissue repair, such as the repair of fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
IMPORTANT SAFETY INFORMATION:
Possible complications following implantation of the GalaFLEX™ Scaffold collection include infection, seroma, pain, scaffold migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, extrusion and recurrence of the soft tissue defect. In pre-clinical testing, the GalaFLEX™ Scaffold collection elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the scaffold was resorbed. For complete prescribing information, including indications for use, warnings and precautions, consult the specific GalaFLEX™ Scaffold product Instructions for Use.
- Preclinical data on file.Results may not correlate to clinical outcomes.
- Deeken CR, Matthews BD. Characterization of the Mechanical Strength,Resorption Properties, and Histologic Characteristics of a Fully AbsorbableMaterial (Poly-4-hydroxybutyrate-PHASIX Mesh) in a Porcine Model of HerniaRepair. ISRN Surg. 2013;2013:238067. Published 2013 May 28. doi:10.1155/2013/238067
- Williams SF, Martin DP, Moses AC. The history of GalaFLEX P4HB Scaffold. AesthetSurg J. 2016;36(suppl 2):S33-S42. doi:10.1093/asj/sjw141
- Obaji S. Why does skin wrinklewith age? What is the best way to slow or prevent this process? ScientificAmerican. September 26, 2005. Accessed February 6, 2024. https://www.scientificamerican.com/article/why-does-skin-wrinkle-wit/
- Choi JW, Kwon SH, Huh CH, Park KC, Youn SW. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19(1):e349-e355.doi:10.1111/j.1600-0846.2012.00650.x
- Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013;5(2):264-270.doi:10.4161/derm.23872
- Levenson SM, Geever EF,Crowley LV, Oates JF 3rd, Berard CW, Rosen H. The healing of rat skin wounds. Ann Surg. 1965;161(2):293-308.doi:10.1097/00000658-196502000-00019
- Vera M. Phases of Wound Healing:The Breakdown. Wound Source. Accessed on Nov 13, 2020 at: https://www.woundsource.com/blog/phases-wound-healing-breakdown
- Teller P, White TK. The physiology of wound healing: injury through maturation. Surg Clin North Am. 2009;89(3):599-610. doi:10.1016/j.suc.2009.03.006
- Wallace HA, Basehore BM,Zito PM. Wound Healing Phases. StatPearls Publishing; 2023. Accessed February 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470443/
- Xue M, Jackson CJ.Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (NewRochelle). 2015;4(3):119-136. doi:10.1089/wound.2013.0485
- Scott JR, Deeken CR,Martindale RG, Rosen MJ. Evaluation of a fully absorbable poly-4-hydroxybutyrate/absorbable barrier composite mesh in a porcine model of ventral hernia repair. Surg Endosc. 2016;30(9):3691-3701. doi:10.1007/s00464-016-5057-9
- GalaFLEX™ Scaffold Instructions for Use.
- Klinge U, Junge K,Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V. Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res. 2002;63(6):765-771.doi:10.1002/jbm.10449
- Halaweish I, Harth K,Broome AM, Voskerician G, Jacobs MR, Rosen MJ. Novel in vitro model for assessing susceptibility of synthetic hernia repair meshes to Staphylococcusaureus infection using green fluorescent protein-labeled bacteria and modern imaging techniques. Surg Infect (Larchmt). 2010;11(5):449-454. doi:10.1089/sur.2009.048
- Engelsman AF, van der Mei HC, Ploeg RJ, Busscher HJ. The phenomenon of infection with abdominal wall reconstruction." Biomaterials. 2007;28(14), 2314-2327.
- Deeken CR, Chen DC,Lopez-Cano M, Martin DP, Badhwar A. Fully resorbable poly-4-hydroxybutyrate(P4HB) mesh for soft tissue repair and reconstruction: A scoping review. Front Surg. 2023;10:1157661.Published 2023 Apr 12. doi:10.3389/fsurg.2023.1157661
- Martin DP, Williams SF. Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Eng J 2003;16(2):97-105.
- Martin DP, Badhwar A, Shah DV, et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J Surg Res. 2013;184(2):766-773.doi:10.1016/j.jss.2013.03.044
- Based on surgeon feedback.