- Overview
- Acute Care Facilities
- Alternate Sites
- FAQs
- Resources
Critical moments demand reliable solutions. That's why BD has invested more than $10 million in 2024 to expand U.S. manufacturing, adding hundreds of millions of units annually to support critical U.S. health care delivery such as hospital procedures, vaccinations, medication preparation and drug delivery to patients.
Choose confidently
We know clinicians have a choice. When making that choice, the details make all the difference. For over 125 years, BD has built a legacy of performance, trust and consistency—providing a comprehensive portfolio of high-quality products more widely used by healthcare professionals than any other brand.
So clinicians can choose confidently.
A legacy of performance and trust
#1 selling needle and syringe portfolio in the U.S.
BD manufactures more needles and syringes than any other brand
BD offers a comprehensive portfolio of safety injection products to fit your specific needs
BD syringes are validated for use on most major infusion pump brands,* including the BD Alaris™ Infusion Pump
*Check with syringe pump manufacturer or syringe pump manual for compatibility of syringe with specific syringe infusion pumps.
Hypodermic solutions you can count on
To deliver quality care 24/7, you need hypodermic products you can depend on every time. BD hypodermic solutions deliver the reliability, compatibility and adaptability you need.
98% of BD hypodermic customers surveyed globally agree that the BD brand stands for consistent, high quality…and that they would recommend BD products to their colleagues.*
*Based on a survey of 368 participants
Supporting supply continuity
Having the right hypodermic solutions on hand when you need them is critical. That’s why we’ve taken multiple significant actions to support reliability and continuity of supply.
$75 million+
investment in Nebraska plants since 2018 to expand injection device capacity
3 billion+
needles and syringes are produced by BD every year for the U.S. market capacity
Click below to view the comprehensive Safety and Conventional Hypodermic SKU updates
Strategically positioned manufacturing to support your needs
More than 1,500 U.S.-based manufacturing employees in Canaan, CT and Columbus, NE
Get to know Dustin #1
Get to know Dustin #2
Get to know Kurt
Get to know Dustin #3
Get to know Jeanine
Get to know Cathy
Get to know Dustin #1
Get to know Dustin #2
Get to know Kurt
Get to know Dustin #3
Get to know Jeanine
Get to know Cathy
-
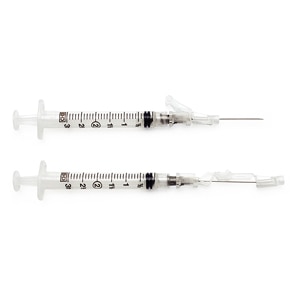
BD® Conventional Needles and Syringes are designed and manufactured to deliver consistent quality and reliable performance.
-
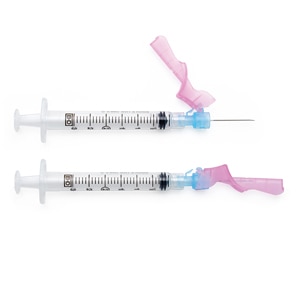
BD SafetyGlide™ Needles have Activation Assist technology, making a safe injection easier and more efficient
-
The BD Eclipse™ Needle is easy to use with minimal training, and supports streamlined and cost-effective option for every practitioner, every day*
-
BD® Blunt Fill and Blunt Filter Needles help reduce the risk of needlestick injuries during medication preparation due to a higher skin penetration force**
-
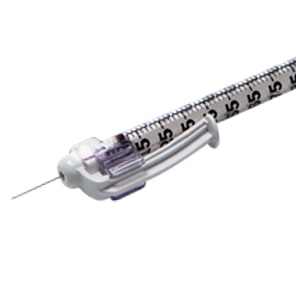
The BD SafetyGlide™ Syringe for TB and Allergy is specifically designed to deliver small doses of medication, while helping keep clinicians and patients safe.
*Within the BD safety hypodermic portfolio
**As compared to a BD 18 G conventional hypodermic needle
BD-113223 (10/25)
Do you know whether your syringe and infusion pump have been validated to function effectively together?
Many factors can influence how a syringe interacts with an infusion pump, potentially impacting patient safety and outcomes.
See how a syringe is validated by a pump manufacturer:
According to the FDA, non-pump manufacturer validated syringes can cause improper pump operation, resulting in:
-
Inaccurate fluid delivery1
-
Insufficient occlusion sensing1
-
Other potential problems1
The FDA also states that lack of flow continuity, with one cause being the use of a non-compatible syringe, can have serious consequences, including:1
By choosing a syringe that has been validated for use by the infusion pump manufacturer, you can trust your infusion system will deliver the precise, reliable performance your patients deserve.
The right syringe can help support safe and effective medication delivery
During pump manufacturer validation, the syringe manufacturer and the infusion pump manufacturer work together to confirm the performance of the syringe and pump as a system. Before you begin an infusion, check to ensure that your syringe has been validated by the pump manufacturer by checking documentation, such as the syringe pump IFU, user manual or compatibility card.
Choose with confidence
The BD® Syringe has been tested and validated for use on most infusion pumps* including BD Alaris™ Infusion Systems. The BD® Syringe is also more widely used by healthcare professionals globally than any other brand.
*Check with the syringe pump manufacturer or IFU for compatibility of the syringe with specific syringe infusion pumps.
The cost of switching to safety-engineered devices (SEDs) from conventional devices isn’t simply a comparison of prices. For needlestick injuries (NSIs), every incident carries potential clinical and emotional burdens for healthcare workers and economic burden for healthcare systems.
Take control of healthcare worker safety
The use of safety-engineered devices (SEDs) has been proven to prevent needlestick injuries.
-

93% reduction in needlestick injuries when safety devices were used. Education and training were key factors5
-

80% of injection/venipuncture administration injuries that could have “probably” or “definitely” been prevented by safety devices6
Designed for your safety
Through our broad portfolio of safety-engineered devices, BD is committed to helping clinicians and institutions meet clinical needs and transform safety at each step of the care continuum.
- U.S. leader of safety injection devices and syringes
- A broad portfolio of safety injection technology
Take the first step toward improved outcomes
The BD Sharps Safety Assesment is designed to help standardize safety device utilization and reinforce best practices through a detailed, actionable road map of BD product recommendations and training—which may help to reduce potential risk factors affecting NSI rates and costs.
-

Proven methodology
-

Proprietary tools
-

Best practices recommendations
- U.S. Food and Drug Administration. Syringe pump problems with fluid flow continuity at low infusion rates can result in serious clinical consequences: FDA Safety Communication. Accessed on July 1, 2020, at: https://www.fdanews.com/ext/resources/files/2016/08/08-25-16-pumpsafetynotice.pdf?1480880246 Archived at https://web.archive.org/web/20161113211840/http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm518049.htm
- Centers for Disease Control and Prevention. 2008 Sharps Injury Prevention Workbook. Accessed on March 15, 2023, at https://www.cdc.gov/sharpssafety/pdf/workbookcomplete.pdf.
- Occupational Safety and Health Administration. United States Department of Labor. Hazard Recognition. Accessed on March 15, 2023, at http://www.osha.gov/bloodborne-pathogens/hazards# .
- Occupational Safety and Health Administration. United States Department of Labor. Bloodborne Pathogens and Needlestick Prevention. Accessed on March 15, 2023, at http://www.osha.gov/bloodbornepathogens#.
- De Carli G, Abiteboul D, Puro V. The importance of implementing safe sharps practices in the laboratory setting in Europe. Biochem Med (Zagreb). 2014;24(1):45-56. Published 2014 Feb 15. doi:10.11613/BM.2014.007.
- Cullen BL, Genasi F, Symington I, et al. Potential for reported needlestick injury prevention among healthcare workers through safety device usage and improvement of guideline adherence: expert panel assessment. J Hosp Infect. 2006;63(4):445-451. doi:10.1016/j.jhin.2006.04.008.
BD-113223 (10/25)
Reliability, safety and efficiency
Whether administering medication or delivering vaccines, you rely on hypodermic needles and syringes multiple times a day, every day. BD is committed to providing high quality hypodermic solutions that deliver the reliable performance you need, with the enhanced safety and efficiency you deserve.
Supporting your needs and advancing supply reliability with strategically positioning manufacturing
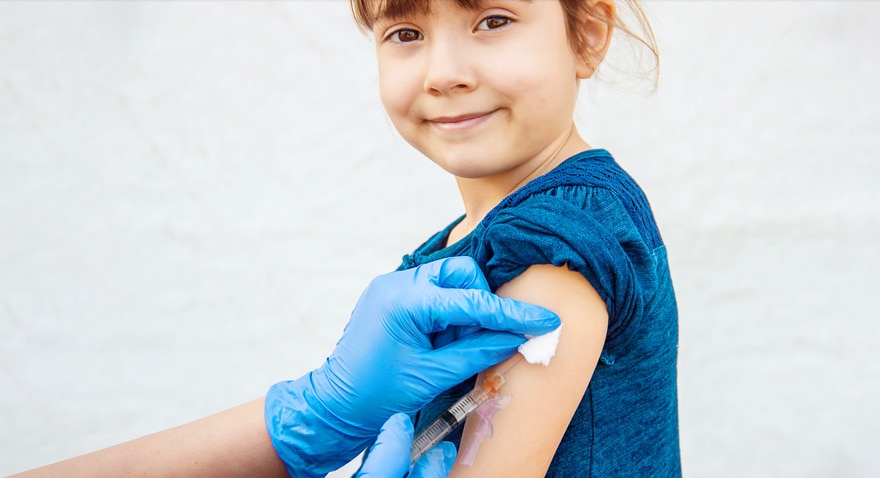
Your partner in vaccinations
BD has been by the side of vaccine developers and healthcare providers for decades, ensuring the availability and safe administration of vaccines from polio and COVID-19 to the seasonal flu.
With a full suite of syringes and safety injection needles designed to help improve the patient experience, you can be confident that there is a BD hypodermic solution to meet your vaccination needs.
-

BD SafetyGlide™ Needles have Activation Assist technology, making a safe injection easier and more efficient
-

The BD Eclipse™ Needle is easy to use with minimal training, and supports streamlined and cost-effective option for every practitioner, every day*
*Within the BD safety hypodermic portfolio
The FDA safety notice does not apply to any BD Syringes. Essentially all hypodermic syringes BD provides to the U.S. healthcare system are manufactured in the U.S. in Nebraska and Connecticut.
The FDA safety notice is regarding plastic syringes manufactured in China. At this time, glass syringes, pre-filled syringes or syringes used for oral or topical purposes are not included.
The FDA safety notice is regarding plastic syringes manufactured in China. At this time, glass syringes, pre-filled syringes or syringes used for oral or topical purposes are not included.
BD has invested $10M to expand manufacturing at our Connecticut and Nebraska plants and remains committed to supporting the U.S. healthcare system to help supply those providers who currently purchase syringes impacted by the FDA communication. BD has increased manufacturing of 1,3,5 and 10 mL syringes to meet demand. We are making every effort to understand the immediate and long-term needs of the U.S. market and we are urging our customers and distributors to place orders as soon as possible.
Pump manufacturers are responsible for determining whether syringes are compatible through a rigorous validation process. Users should check with the syringe pump manufacturer or syringe pump manual for compatibility of syringe with specific syringe infusion pumps.
BD offers the needle lengths clinicians use most, and those that align with industry guidelines. The needle lengths clinicians rely on, and those needed most, will remain available, including: 0.375”, 0.5”, 0.625”, 1”, 1.5” and 2”.
The optimized BD hypodermic portfolio includes needle sizes that meet a variety of clinical applications, including: 16 G, 18 G, 21 G, 23 G, 25 G, 27 G and 30 G needles.
Streamlined needle lengths and gauges, which may help with:1,2
- Standardization
- Streamlined ordering processes
- Improved operations
- Reduced storage costs
- Rosser M. Advancing health system integration through supply chain improvement. Healthc Q. 2006;9(1):62-66.
- McKesson. Leveraging Product Standardization for Health System Efficiency. February 20, 2018. https://mms.mckesson.com/Blog/Leveraging-Product-Standardization-for-Health-System-Efficiency/. Accessed July 27, 2021.
BD-113223 (07/24)