BD Nexiva™ Closed IV Catheter System is not just a catheter. BD Nexiva™ Closed IV Catheter System is an all-in-one PIVC, combining four individual devices in one: IV catheter, extension set, stabilization device, and needle-free connector/s. It is clinically demonstrated to have longer dwell times – dwelling up to 144 hours – and reduced complications versus an open system.*,1,2


- Overview
- Best Practices
- Products & Accessories
- EIFU & Resources
BD Nexiva {*} has been shown to reduce costs and reduce treatment delays in clinical studies.**,1,3
Dwells longer
In a randomized study comparing the BD Nexiva {*} to an open catheter system*, among peripheral IV catheters in place for over 24 hours, the median dwell time for BD Nexiva™ {*} was up to 144 hours versus 96 hours for the open system.*,1
Preserves sites
BD Nexiva {*} is an all-in-one PIVC shown to preserve sites for longer.†,1,3
By choosing the BD Nexiva {*}, you can reduce the risk of complications and blood exposure as well as preserve sites.**,1,3
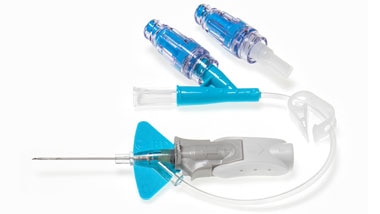
BD Nexiva {*} with integrated extension tubing and stabilization platform reduces the risk of dislodgement and related complications.‡,1,3
The BD Nexiva {*} with the built-in stabilization platform reduces dislodgement by 84%.‡,3
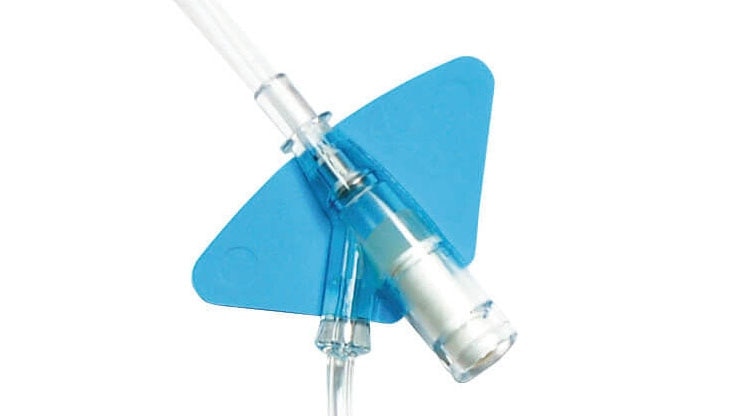
Built-in stabilization platform‡
BD Nexiva {*} with an integrated extension tubing and stabilization platform is designed to reduce manipulation and movement at the site and has been shown to reduce dislodgement and phlebitis.‡,1,3
First attempt insertion
BD Instaflash™ Needle Technology incorporates a notched needle, which is designed to improve first-stick success and reduce painful hit-and-miss insertions.4,5

Incorporates a notched needle designed to provide immediate visual confirmation of vessel entry.5,6
The BD Nexiva {*} demonstrated a 29% reduction in phlebitis rates when compared to an open system.*,1

BD Vialon™ Catheter Material
Proprietary BD Vialon™ Catheter Material softens, enabling longer dwell time and reducing the chance of phlebitis up to 69%.§,7-9
BD Nexiva™: Traditional vs closed IV catheter insertion
Catheter stabilization is recognized as an intervention to decrease the risk for phlebitis, catheter migration and dislodgement and may be advantageous in preventing catheter-related bloodstream infections (CRBSIs).3
Recommend limiting the use of add-on devices to reduce the potential for contamination, additional manipulation, and disconnection.5
98% reduced blood exposure during insertion due to the BD Nexiva IV catheter preassembled system.2*
Clinically demonstrated to reduce accidental dislodgement,2‡ meeting Infusion Nursing Society standards4 and CDC guidelines5 for catheter stabilization.
In a clinical study, results demonstrated a significant reduction in the rate of phlebitis (grade 2 or higher), PIVC-related complications, and infiltration in the closed system versus the open system group.1
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Tamura N, Abe S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements can be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- van Loon FHJ, Puijn LAPM, Houterman S, et al. Development of the A-DIVA Scale, A clinical predictive scale to identify difficult intravenous access in adult patients based on clinical observations. Medicine. 2016;95(16):e3428. Doi: 10.1097/MD.0000000000003428.
- van Loon FHJ, Timmerman R, den Brok GPH, et al. The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: block-randomized trial. J Vasc Access. 2021;23(2):295-303. doi:10.1177/1129729821990217.
- Seetharan AM, Raju U, Suresh K. A randomized controlled study to compare first stick success with Instaflash Technology: The FIRSST study. J Vasc Access. 2022. doi: 10.1177/11297298221080369.
- Kus B, Buyukzilmaz F. Effectiveness of vialon biomaterial versus teflon catheters for peripheral intravenous placement: A randomized clinical trial. Jpn J Nurs Sci. 2020;e12328. https://doi.org/10.1111/jjns.12328.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Annals of Internal Medicine. 1991;114:845-854.
- Gaukroger PB, Roberts JG, Manners TA. Infusion thrombophlebitis: a prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesthesia and Intensive Care. 1988;16:265-271.
Notes
*With an PTFE catheter.
**Compared to an open system.
†Up to 144 hours compared to 96 hours with an open system with a PTFE catheter.
‡BD NexivaTM Catheter System with 3MTM TegadermTM IV Securement Dressing compared to B. Braun Introcan Safety® catheter with Statlock® IV Ultra stabilization device and non-bordered dressing.
§Compared to an FEP catheter.
BD-3595 (10/23)