BD® PosiFlush™ Prefilled Flush Syringes
When it's going into your patient's body, quality matters


- Overview
- The BD PosiFlush™ Difference
- Best Practices
- Products & Accessories
- EIFU & Resources
A routine task with big impact
While flushing may seem like just another task, it’s an important part of maintaining patency of the line and reducing the risk of complications, including occlusion, or premature catheter failure. In addition, flushing before and after medication is a strongly recommended step in medication delivery, helping prevent contact between incompatible medicines and ensure that the entire dose of medication is delivered.
BD PosiFlush™ Prefilled Flush Syringes are designed with quality and improved outcomes in mind
INS Guidelines recommend the use of "commercially manufactured prefilled flush syringes (when available) to reduce the risk of catheter-associated bloodstream infection (CABSI) and device failure, save time for syringe preparation, and aid optimal flushing technique and objectives."1
A comprehensive prefilled flush syringe portfolio
Featuring a broad selection of saline and heparin prefilled products in sterile path and externally sterile packaged syringe formats, the BD PosiFlush™ Prefilled Flush Syringe portfolio provides flushing solutions to help meet the needs of clinicians and patients.
BD PosiFlush™ SP Saline Prefilled Syringe
BD PosiFlush™ Sterile Path (SP) Saline Prefilled Syringes are intended to be used for the flushing of indwelling vascular access devices to help support maintaining patency as part of medication delivery. They are designed for use outside of sterile areas only.
BD PosiFlush™ SF Saline Prefilled Syringe
BD PosiFlush™ Sterile Field (SF) Saline Prefilled Syringes are intended to be used only for the flushing of indwelling vascular access to maintain patency and support medication delivery. They are terminally sterilized in their peel pouch, enabling them to be aseptically presented to a sterile field.
BD PosiFlush™ Prefilled Heparin Lock Flush Syringes
BD PosiFlush™ Prefilled Heparin Lock Flush Syringes may help maintain catheter patency by locking vascular access devices, when used in accordance with your institution access maintenance protocol. They are available in two concentrations—10 USP units per mL and 100 USP units per mL— to help support your catheter maintenance practice.
*In vitro test results may not be predictive of clinical performance. Demonstrated reduction of the most common causative agents of CRBSI including Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Candida glabrata, Candida albicans and Acinetobacter baumannii.
- Nickel B, Gorski L, Kleidon T, et al. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024;47(2S Suppl 1): S126. doi:10.1097/NAN. 0000000000000532.
Not all prefilled flush syringes are created equal
Every time you flush a patient's line, you're injecting saline directly into their bloodstream. Quality defects and bacterial contamination in prefilled flush syringes have seriously harmed patients:1-3
Catheter-related infections4
Spinal meningitis4
Permanent brain damage4
11 deaths, including a 2-year old child1,5
With BD PosiFlush™ Prefilled Flush Syringes, you can flush with confidence

Safety matters
100% TOUCH-FREE
100% touch-free manufacturing,6 ensuring the first person to touch the syringe is the clinician
MULTI-STEP INSPECTION
100% of BD PosiFlush™ Prefilled Flush Syringes are inspected using a rigorous, multi-step, end-to-end inspection process to meet the highest quality standards and safety for our customers and patients

Experience matters
20+ YEARS
in the market as a partner, supporting the needs of patients globally
18 BILLION+ SYRINGES
produced and sold to date—more than any other prefilled flush brand
13,000+ CUSTOMERS
around the world use and trust BD PosiFlush™ Prefilled Flush Syringes

Reliability matters
BD DELIVERED FOR OUR CUSTOMERS
when market changes necessitated additional support by increasing BD PosiFlush™ unit production more than 25% from 2020-2022
3-YEAR SHELF LIFE
enabling facilities to plan ahead and manage inventory
6 HEALTH ORGANIZATIONS'
flushing best practice guidelines met (INS, RNC, NICE, epic 3, INICC, ISMP)7-13
INS: Infusion Nurses Society, RCN: Royal College of Nursing, NICE: National Institute for Health and Care Excellence, INICC: International Nosocomial Infection Control Consortium, ISMP: Institute for Safe Medication Practice
BD is meeting our customers' needs of responsive and timely delivery, supported by three manufacturing plants and our global network of distribution centers
ISO-compliant quality management systems
with relevant standards for quality assurance14 and environmental best practices15
Published clinical studies
BD PosiFlush™ Prefilled Flush Syringes are supported by 20+ clinical studies—more published clinical study support than any other product in the prefilled syringe category*
*Based on a Pubmed literature search of key saline flush competitors as of July 2023
At BD, we understand that the health of the planet is linked to the health of people, and reducing our impact on the environment supports our purpose of advancing the world of health™.
Below are just a few examples of our efforts to minimize the BD PosiFlush™ Prefilled Flush Syringe portfolio's impact on the planet.
-
Sustainable manufacturing
All BD PosiFlush™ manufacturing plants are ISO 14001 certified to ensure sustainable best practices in manufacturing.
-
CO2 emissions
We are optimizing the BD PosiFlush™ supply chain and distribution strategy to reduce CO2 emissions.
-
Products
BD PosiFlush™ Prefilled Flush Syringes are offered in a variety of syringe sizes to support utilization of the proper size, given the situation and patient. Utilizing smaller sized (3mL /5mL) BD PosiFlush™ Prefilled Flush Syringes helps reduce disposal waste.
-
Manufacturing
From FY19-FY22, the BD Columbus manufacturing plant, where BD PosiFlush™ Prefilled Flush Syringes are produced, reduced its greenhouse gas (GHG) emissions on an absolute basis by 45%, and is investigating further opportunities for reduction.
- Jewett C, Nguyen D. Timeline: How Contaminated Syringes Got From Factory to Patients. June 6, 2009. Accessed May 2, 2023 at https://www.propublica.org/article/timeline-am2pat
- U.S. Food and Drug Administration. Cardinal Health Recalls Monoject Saline Flush Prefilled Syringes for Risk of Air Re-entering Syringe Leading to Air Embolism. Accessed on May 2, 2023, at https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/cardinal-health-issues-nationwide-recall-select-monojecttm-flush-prefilled-saline-syringes#:~:text=On%20August%204%2C%202021%2C%20Cardinal,the%20air%20has%20been%20expelled.
- Hudson MJ, Park SC, Mathers A, et al. Outbreak of Burkholderia stabilis Infections Associated with Contaminated Nonsterile, Multiuse Ultrasound Gel - 10 States, May-September 2021. MMWR Morb Mortal Wkly Rep. 2022;71(48):1517-1521. Published 2022 Dec 2. doi:10.15585/mmwr.mm7148a3.
- Massoomi F, Arruda J. A Closer Look at Prefilled Flush Syringe Safety. Pharmacy Practice News. February 15, 2018. Accessed on May 19, 2023, at https://www.pharmacypracticenews.com/Clinical/Article/02-18/A-Closer-Look-at-Prefilled-Flush-Syringe-Safety/46801?sub=C63733F385E1FD4090EBC251E2F1174E9B162D4541C3E518622EA66D7AFC67F&enl=true%3Fses%3Dogst
- Centers for Disease Control and Prevention. Multistate Outbreak of Burkholderia cepacia Bloodstream Infections Associated with Contaminated Prefilled Saline Flush Syringes. 2017. Accessed March 7, 2024 at https://www.cdc.gov/hai/outbreaks/b-cepacia-saline-flush/index.html
- Brown K, Graf LM, Guyader MJ, et al. Technical Report No. 48 Moist Heat Sterilizer Systems: Design, Commissioning, Operation, Qualification and Maintenance. Bethesda, Maryland, United States: Parenteral Drug Association; 2010.
- Nickel B, Gorski L, Kleidon T, et al. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024;47(1S Suppl 1):S1-S285. doi:10.1097/NAN.0000000000000532.
- Denton A, Bodenham A, Conquest A, et al. Royal College of Nursing. Standards for Infusion Therapy, 4th Edition. https://www.rcn.org.uk/-/media/royal-college-of-nursing/documents/publications/obselete/005704.pdf?la=en. Published December 12, 2016. Accessed June 2, 2021.
- Loveday HP, Wilson JA, Pratt RJ, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86 Suppl 1:S1-S70. doi:10.1016/S0195-6701(13)60012-2
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: Device-associated module. Am J Infect Control. 2020;48(4):423-432. doi:10.1016/j.ajic.2019.08.023.
- National Patient Safety Agency. NPSA Alert - Promoting safer use of injectable medicines (NPSA 20). Published March 28, 2007. Updated August 22, 2018. Accessed October 4, 2019 at https://www.sps.nhs.uk/articles/npsa-alert-promoting-safer-use-ofinjectionmedicinesnpsa-20-2007/
- The Institute for Safe Medication Practices. ISMP Safe Practice Guidelines for Adult IV Push Medications. ISMP. Published July 14, 2015. Accessed October 20, 2020 at http://www.ismp.org/sites/default/files/attachments/2017-11/ISMP97-Guidelines-071415-3.%20FINAL.pdf.
- The Joint Commission. National Patient Safety Goals Effective July 2020 for the Hospital Program. The Joint Commission. Accessed October 20, 2020 at http://www.jointcommission.org/-/media/tjc/documents/standards/national-patientsafetygoals/2020/npsg_chapter_hap_jul2020.pdf. Published March 26, 2020.
- International Organisation for Standardisation (ISO) 13485 standard on Medical devices – Quality management systems – Requirements for regulatory purposes.
- International Organisation for Standardisation (ISO) 14001 standard on Medical devices – Environmental best practices – Requirements for regulatory purposes.
The importance of flushing and locking
Flushing is the act of moving fluids, medications, blood and blood products out of the vascular access device into the bloodstream. Locking is the instillation of a solution into a vascular access device.1
Flushing is a critical part of catheter care and maintenance and medication delivery. INS practice recommendations support flushing before and after medication delivery.1
Why is flushing and locking important?
INS 2024 Standards of Practice recommend using a single-use, commercially prepared, prefilled syringe of appropriate solution to flush and lock vascular access devices.1
SASH Protocol
How closely do your routines align
INS 2024 Standards of Practice emphasize that clinicians should:1
-

“Use a new, unopened, single-use commercially available prefilled syringe”
For each flush1—never re-use a syringe
-

Flush before and after medication administration1
Not adhering to flushing best practice can significantly impact patient outcomes.
-
Re-using a prefilled flush syringe increases the risk of contamination and CLABSI1
-
Flushing after each medication administration reduces the risk of contact between incompatible medications with the potential to cause patient harm
-
Estimated $45K cost per patient to treat CLABSI3
-
Between 12–25% mortality rate with CLABSI4
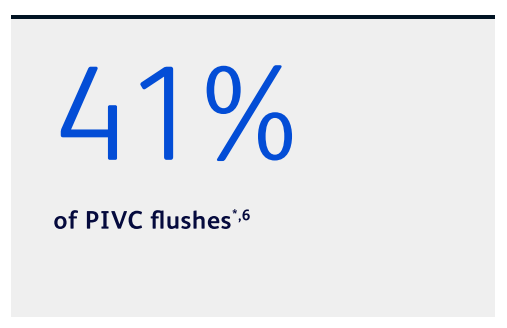
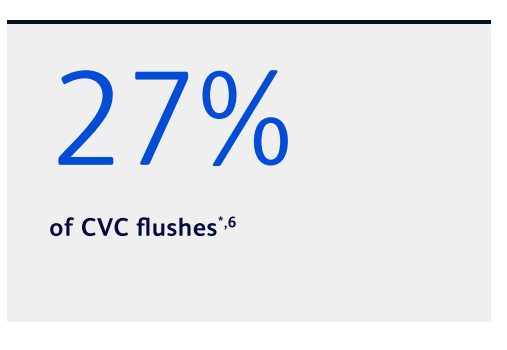
Nurses report using the same syringe for both pre- and post-medication administration flushes in:
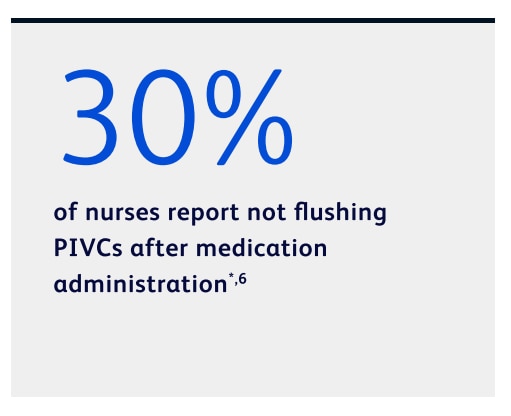
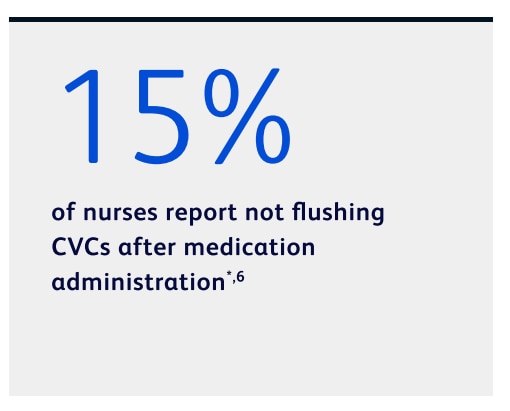
Many nurses report not flushing for post-medication administration6
*Based on a clinician survey conducted in 2020
Using a new, unopened, single-use, commercially available prefilled flush syringe for flushing before and after medication administration:
-
Reduces the risk of touch contamination of the prefilled flush syringe tip and transfer of that contamination to the vascular access device and patient.1
-
Helps avoid potential dangerous interaction of incompatible medications and negative patient impacts.5
- Nickel B, Gorski L, Kleidon T, et al. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024;47(1S Suppl 1):S265. doi:10.1097/NAN.0000000000000532.
- Goossens GA. Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs Res Pract. 2015;2015:985686. doi:10.1155/2015/985686.
- Zimlichman E et al. JAMA Intern Med. 2013:173(22):2039-2046.
- Centers for Disease Control and Prevention (CDC). MMWR. 2011;60(8):243-248.
- Grissinger M. Pa Pat Saf Advis. 2016 Dec;13(4):137-148.
- Survey: IPSOS—PosiFlush AAU Final Quant Report, Jan 2020. Data on file.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.