BD® Port Access Kits and Needles
Convenient, all-in-one sterile solutions for port access procedures—designed to promote consistency of care at each step


- Overview
- Products & Components
- EIFU & Resources
Protect what matters. Sterility. Standards. Safety.
When patient safety is involved, port access is never just another task. It requires attention to detail, and consistency at each step.
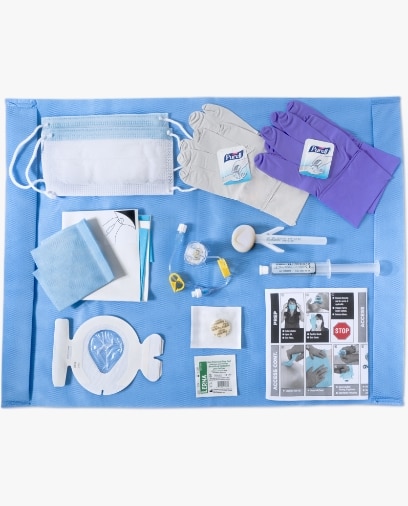
BD® Port Access Kits and AllPoints™ Port Access Systems combine all essential port access procedure components from the Port Access market leader into one sterile, streamlined and convenient kit—designed to reduce port access procedure variability and promote standard compliance for each patient, in each port access procedure.
AllPoints™ Brochure
An all-in-one convenient solution with components organized to promote standardized, sterile “best practices” for port access-related procedures.

Port Access Portfolio Brochure
BD is the Port Access market leader, and is the only major manufacturer to offer an all-inclusive port access kit that integrates the infusion needle directly within the kit.
BD® Port Access Kits and AllPoints™ Port Access Systems are designed to be a simple and effective way to standardize the port access procedure process and to promote compliance with guidelines for sterile port access.
Provides the convenience of combining all necessary port access procedure components in a single package.
A procedural checklist is included in every kit to facilitate sterile technique, guiding the clinician through the procedural steps.
-
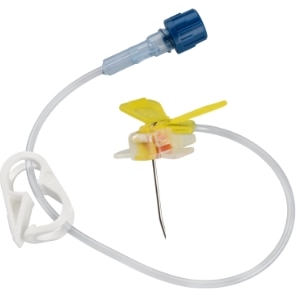
MiniLoc™ Safety Infusion Set
The safety mechanism on the BD MiniLoc™ Safety Infusion Set is an easy to use, single step safety activation. The needle point safety is designed to help reduce the risk of needlestick injuries.
View products
-
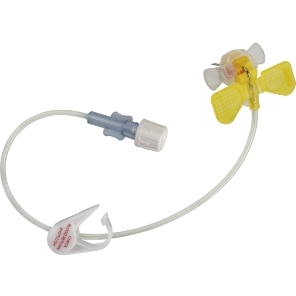
PowerLoc™ Safety Infusion Set
The BD PowerLoc™ Safety Infusion Set is a winged power injectable port access needle with a passive needle tip safety designed to protect against healthcare worker needlestick injuries.
View products
-

SafeStep™ Huber Needle Set
The SafeStep™ Huber Needle Set increases access site visibility due to a clear plastic base. The BD SafeStep™ safety mechanism requires no additional technique when removing the needle from a port.
View products
-
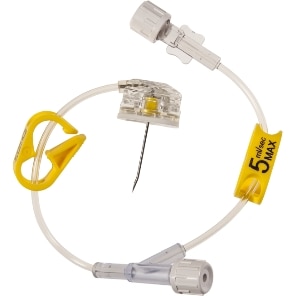
PowerLoc™ Max Safety Infusion Set
The PowerLoc™ Max Power-Injectable Infusion Set is a non-winged power-injectable port access needle with a passive needle tip safety designed to help protect against healthcare worker needlestick injuries.
View products
-

BD Amanda™ Port Stabilizer
Designed to aid access when inserting the infusion needle into single-port devices. INS guidelines suggest the use of needle insertion assistive devices may improve first- attempt success.
View products
-

Sentrinex™ 3D Port Dressing (stand-alone)
Created specifically for port access, this 3D domed dressing is designed to fit over most needle access devices and lay flat against the skin.
View products
-

GuardIVa™ Antimicrobial Hemostatic IV Dressing
The chlorhexidine gluconate (CHG) in the GuardIVa™ Antimicrobial Hemostatic IV Dressing is a well-known antiseptic agent with broad spectrum antimicrobial and antifungal activity.
The chlorhexidine gluconate (CHG) in the GuardIVa™ Antimicrobial Hemostatic IV Dressing is a well-known antiseptic agent with broad spectrum antimicrobial and antifungal activity.
View products
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
BD-66999 (09/22)