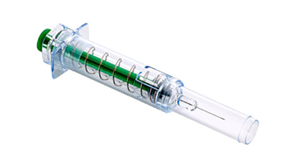
BD Preventis™ Needle Shielding System
BD tools for anticoagulant combination product developers

- Overview
- Expertise
- Products & Accessories
- Resources
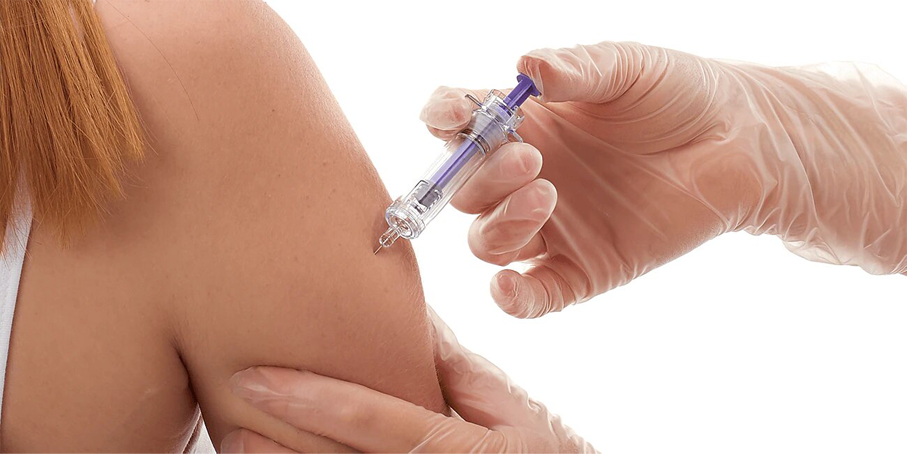
- The BD Preventis™ Needle Shielding System when assembled with a Glass Prefillable Syringe1 is intended for the subcutaneous administration of anticoagulants in both clinical and non-clinical environments.
- BD Preventis™ enables one-handed, controlled activation that automatically shields the needle; and nurses who received instructions were able to successfully recognise the activation of the Needle Shielding System.2
- Intended for use by Healthcare Workers and Caregivers,1 BD Preventis™ comes with technical documentation and support3 and is compatible with BD Hypak™ SCF™ Glass PFS.4
BD PreventisTM Needle Shielding System Features
- BD PreventisTM Plunger rod available - available in different colors for customisation
- Active Needle Shielding System - an affirmative activation step is required to deply the needle shield
- Design to allow syringe scale visualisation5
- Compatible with 0,5 mL and 1 mlL BD HypakTM SCFTM Glass Prefillable Syringes4
BD PreventisTM Needle Shielding System Benefits
- Enables one-handed2 , controlled activation that automatically shields the needle Nurses who received instructions were able to successfully recognise the activation of the needle shielding system2
- Comes with technical documentation and support3
- Intended users1 : Healthcare Workers and Caregivers
- Intended use: when assembled with a Glass Prefillable syringe (PFS)1 . Subcutaneous administration of anticoagulants in both clinical and non-clinical environment.
- Compatible with BD Hypak™ SCF™ Glass PFS4
BD does not make any claim with regards to BD Preventis™ compliance to sharps injury requirements and its ability to meet safety legislations requirements.
- RMSR 0069_2011 Rev 02 [Internal report]. Pont de Claix, FR, Becton, Dickinson and Company.
- Out of 576 uses, 93.8% of nurses say they activate BD PreventisTM with one hand, BDPS-01-13, [Internal report], Pont-de-Claix, FR, Becton Dickinson and Company, July 2001.
- BD provides the operational sequence of use for the product to customer.
- SD20190231 rev3.0, [Internal report], Pont-de-Claix, FR, Becton Dickinson and Company.
- DVTR20200072, [Internal report], Pont-de-Claix, FR, Becton Dickinson and Company.
Our expertise in BD PreventisTM Needle Shielding System
- Established on the market since 2003
- More than 1.5 billion units sold1
- Samples available on demand
- Commercial availability to be evaluated against requirements
BD does not make any claim with regards to BD Preventis™ compliance to sharps injury requirements and its ability to meet safety legislations requirements.
- BD analysis [Internal report]. Pont de Claix, FR, Becton, Dickinson and Company; 2021.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.