Indicated for the use in peripheral arteries for:
- Native Bypass
- Artificial Bypass
- Stent Grafts
- In-Stent Restenosis
- Atherectomy
- Thrombectomy
Refined Atherectomy. Built to Remove.
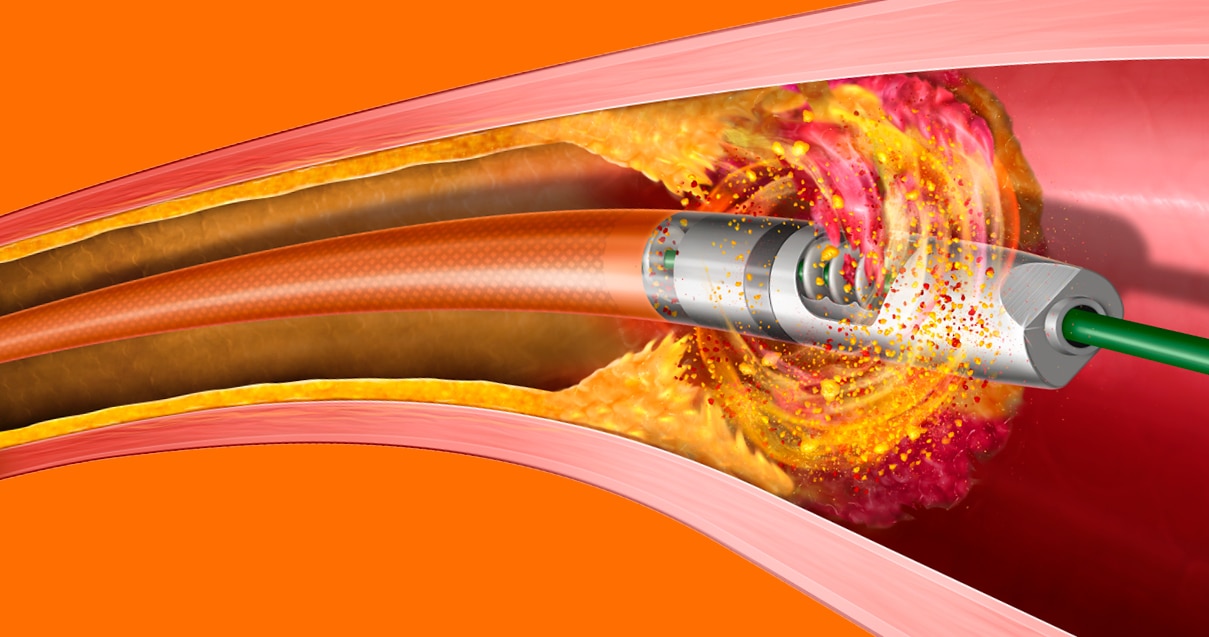

The Rotarex™ Atherectomy System is designed to efficiently remove both plaque and thrombus by utilizing three distinct mechanisms of action to treat PAD lesions including in-stent restenosis. The device modifies, excises, and aspirates complex lesions with mixed plaque morphology with two different size options—6F & 8F.

We understand that anything that can help to save time and space, and reduce complexity in the lab is essential. The Rotarex™ Atherectomy System is simple to set up and easy to use, with a small plug-and-play capital component and reusable handle that is easily draped. Additionally, the Rotarex™ Atherectomy System does not require any warm up, infusion or catheter clean out.

A meta-analysis of 8 clinical studies comprising data obtained from 2,107 patients studied from 2002 to 2015 was performed to:
| Measure/Outcomes | Mean (95% CI) |
| Age | 68.1 (66.4, 69.7) |
| Gender (% Male) | 65.3% (60.6%, 70.0%) |
| Rotarex™ Device Treatment Time (minutes) | 3.0 (1.3, 4.7) |
| Lesion Length (mm) | 153.96 (115.24, 192.69) |
| Technical Success | 95.8% (94.3%, 97.3%) |
| Clinical Success | 79.9% (75.2%, 84.5%) |
| TLR at 6 Months | 7.8% (1.0%, 14.5%) |
| TLR at 12 Months | 11.3% (7.4%, 15.3%) |
| Restenosis at 6 Months | 16.9% (0.0%, 35.3%) |
| Restenosis at 12 Months | 35.5% (19.6%, 51.4%) |
| Measure/Outcomes | Mean (95% CI) |
| ABI at 6 Baseline | 0.33 (0.18, 0.47) |
| ABI at 6 Months | 0.83 (0.78, 0.88) |
| ABI at 12 Months | 0.77 (0.71, 0.82) |
| Rutherford Score at Baseline | 3.54 (3.42. 3.67) |
| Rutherford Score at 6 Months | 1.51 (1.03, 2.00) |
| Rutherford Score at 12 Months | 2.13 (1.83, 2.42) |
| Procedure-Related Dissection | 5.9% (3.3%, 8.6%) |
| Procedure-Related Embolization | 7.5% (4.7%, 10.4%) |
| Procedure-Related Perforation | 2.2% (0.9%, 3.4%) |
| Procedure-Related Pseudo-Aneurysm | 1.5% (0.6%, 2.4%) |
| Procedure-Related Abrupt Reocclusion | 2.4% (1.0%, 3.7%) |
64-year-old male patient presented with left-sided CLI. Over the preceding four months the patient experienced left-sided rest pain and despite receiving best medical treatment, developed a dry, non-healing ulcer of the toe. Puncture of the right groin and cross-over approach, demonstrated a very long, 31 cm, TASC D, femoropopliteal CTO on angiogram. The SFA occlusion was recanalized with a wire intraluminally, followed by 3 passes of a 6F Rotarex S™ Atherectomy Catheter, after which 3 PTAs resulted in a completely restored flow. The patient remained asymptomatic after 18 months.
BD is proud to offer you this exclusive learning opportunity to further support your Peripheral Arterial Disease (PAD) education and training with the use of our Rotarex™ Rotational Excisional Atherectomy System. We are committed to making your educational learning with BD Peripheral Intervention (BDPI) a success.
Faculty are available to answer questions about the Rotarex™ Rotational Excisional Atherectomy System, such as: Case/Patient Selection, Device Set Up, Recommendations for Device Use, or Discuss Your Experience With An Expert.
1. Rotarex™ Atherectomy System eIFU
2. All case images are courtesy of the various clinicians

NEWS
Leipzig’s Experience Using Rotarex™ Rotational Excisional Atherectomy for In-Stent Reocclusion in Peripheral Arterial Occlusive Disease
PRESS RELEASE
BD Announces 510(k) Clearance of Expanded Indications for the Rotarex™ Atherectomy System

NEWS
Rotational Excisional Atherectomy With the Rotarex™ Atherectomy Device – Miguel Montero-Baker, MD
AMP 2020
Refining Atherectomy in Challenging Lesions with Mixed Plaque Morphology
AMP 2020
Atherectomy with Thrombectomy: The Rotarex Rotational Excisional Atherectomy System

Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-27143v5