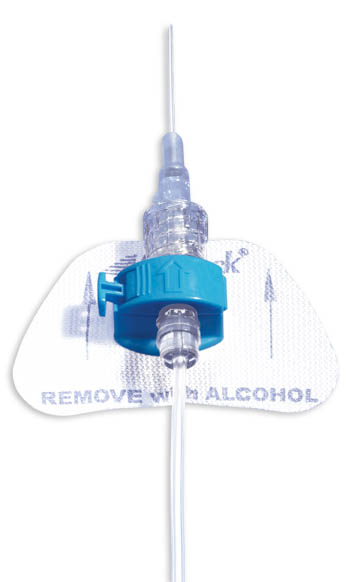
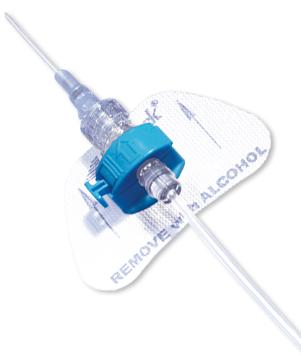
- The color-coded blue retainer is designed for patient safety.
- The precision molded, ergonomic retainer can be opened and closed while in place, allowing for tube set changes whenever necessary.
- Unique entry angle of retainer is designed to prevent catheter kinking, which could lead to occlusion.
- The StatLock™ IV Plus Stabilization Device fits adult and pediatric (over 18 months) patients.
- Breathable anchor pad made from nonabsorbent tricot polyester.
- Sterile, DEHP-free and latex-free.
StatLock™ Stabilization Devices are a more effective alternative to tape in helping improve clinical outcomes, quality of care and economic efficiencies. The StatLock™ IV Plus Stabilization Device offers both a patented luer lock extension set (for universal compatibility with all peripheral IV catheters) and clamshell retainer for ease of use. Available in adult and pediatric sizes.