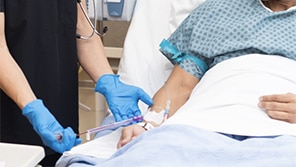
The PIVO™ Pro Blood Collection Device has been shown in clinical and healthy human studies to provide reliable, high-quality blood draws from peripheral IV catheter lines.*,2 In addition to providing high-quality blood draws with low rates of hemolysis, the PIVO™ Pro Blood Collection Device may have a positive impact on patient experience by reducing repetitive needlesticks and redraws that may result in care delays.12
PIVO™ Needle-free Blood Collection Device—compatible with 22 G IVs and larger
- Overview
- Specifications
- eIFUs and Resources
- FAQ
- Product Notifications
Transform the care experience with longer-lasting peripheral IV catheters that enable needle-free blood draws1,2
The PIVO™ Pro Needle-free Blood Collection Device is designed to achieve the first and only compatibility with integrated and long peripheral IV catheters, including the new Nexiva™ Closed IV Catheter System with NearPort™ IV Access in addition to traditional short IV catheters.
BD is transforming clinical workflows and their outcomes1,2 by combining the proprietary technologies of closed IV catheter systems that have shown to improve dwell times and reduce complications with a needle-free blood collection device to overcome traditional IV and blood collection challenges.2
- Humphrey GB, Boon CMJ, van Linden van den Heuvell GFEC. Van de Wiel HBM. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics. 1992;90(1):87-91.
- Hambleton VL, Gomez IA, Andreu, FAB. Venipuncture versus peripheral catheter: do infusions alter laboratory results? JEN. 2014;40(1):20-26.
- Halm M, Gleaves M. Obtaining blood samples from peripheral intravenous catheters: best practice? Am J Crit Care. 2009;18 474-478 10.4037/ajcc2009686
- Fradet C, McGrath PJ, Kay J, Adams S, Luke B. A prospective survey of reactions to blood tests by children and adolescents. Pain. 1990;40:53-60.
- Twibell KR, Hofstetter P, Siela D, Brown D, Jones HM. A comparative study of blood sampling from venipuncture and short peripheral catheters in pediatric inpatients. J Infus Nurs. 2019;42(5):239. do1:10.1097/NAN.0000000000000338
- Cadacio C, Nachamkin I. A novel needle-free blood draw device for sample collection from short peripheral catheters. JIN. 2017;40(3):156-162.
- Pendleton B, LaFaye R. Multicenter study of needle-free blood collection system for reducing specimen error and intravenous catheter replacement. J Healthc Qual. 2022;44(2):e24-e30. doi:10.1097/JHQ.0000000000000331
- Webster A. Easing patient fears can raise HCAHPS scores. Healthleaders Media. 2011
- Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable; peripheral IV catheter failure: Infus Nurs Society. 2015;38(3):189-203.
- Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013;46:1175-1179. doi: 10.1016/j.clinbiochem.2013.06.001
- Taddio A, Ipp M. Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807-4812. doi: 10.1016/j.vaccine.2012.05.011
- Gagne P, Sharma K. Relationship of common vascular anatomy of cannulated catheters. Int J of Vas Med. 2017:5157914. doi:10.1155/2017/515794.
- Adams S, Toroni B, Lele M. Effect of the PIVO device on the procedure of phlebotomy from peripheral IV catheters. Nurs Res Pract. 2018:7380527. doi:10.1155/2018/7380527
- Natali R, Wand C, Doyle K, Noguez JH. Evaluation of a new venous catheter blood draw device and its impact on specimen hemolysis rates. Pract Lab Med. 2018;10:38-43. doi:10.1016/j.plabm.2018.01.002
- Mulloy DF, Lee SM, Gregas M, Hoffman KE, Ashley SW. Effect of peripheral IV based blood collection on catheter dwell time, blood collection, and patient response. Appl Nurs Res. 2018;40:76-79. doi:10.1016/j.apnr.2017.12.006
- Kuriakose L. Decreasing central line associated bloodstream infection through limiting the use of central venous catheters for routine blood draws. J Dr Nurs Pract. 2020:13(2):173-183.doi:10.1891/JDNP-D-19-00071
- Mannocci A, De Carli G, Di Bari V et al, How much do needlestick injuries cost? A systematic review of the economic evaluations of needlestick and sharps injuries among healthcare personnel. Infect Control Hosp Epidemiol. 2016;37(6):635-646. Doi:10.1017/ice.2016.48
- Betts D, Balan-Cohen A, Shukia M, Kumar N. The value of patient experience. Deloitte. Published 2021. Accessed February 1, 2022 at https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-dchs-the-value-of-patient-experience.pdf
- Duffy B. Driving practice growth by restoring humanity to healthcare. Medical Economics. 2015, February.
- Goel D, Smitthimedhin A, Yadav B, et al. Ultrasound-detected venous changes associated with peripheral intravenous placement in children. JAVA. 2020;25(1)36-42.
- Matthews EE. Sleep disturbances and fatigue in critically ill patients. AACN Adv Crit Care. 2011:22(3):204-224. doi:10.1097/NCI.0b013e31822052cb
- McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv. Nurs. 2019;75:30-42
- Sharma SK, Kant R, Kalra S, Bishnoi R. Prevalence of primary non-adherence with insulin and barriers to insulin initiation in patients with type 2 diabetes mellitus – An exploratory study in a tertiary care teaching public hospital. Eur Endocrinol. 2020:16(2):143-147. doi:10.17925/EE.2020.16.2.143
- Ibrahim I, Yau YW, Ong L, Chan YH, Kuan WS. Arterial puncture using insulin needle is less painful than with standard needle: a randomized crossover study. Acad Emerg Med. 2015;22(3):315-320. doi:10.1111/acem.12601
- Gill H, Prausnitz M. Does needle size matter? J Diabetes Sci Technol 2007;1(5):725-729
*As compared to short peripheral catheters.
**Compared to a traditional blood draw.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-66490 (09/22)
GTIN - Case
20850984007038
200
GTIN - Shelfpack
10850984007031
50
GTIN - Each
00850984007034
1
DEHP is not part of the Material Formulation
DEHP is not part of the material formulation
Total Shelf Life
3 Years
Legal Manufacturer's Address
Velano Vascular, San Francisco, CA
Quantity - Each
1
Quantity - Box
50
Quantity - Case
200
Sterilization Method
Gamma
Length
Inner Tube Length 5.85 inches
Material
Inner Tube Material-Polyimide
Color
Blue
GTIN
| GTIN - Case | 20850984007038 | 200 |
| GTIN - Shelfpack | 10850984007031 | 50 |
| GTIN - Each | 00850984007034 | 1 |
FDA, Premarket Approval, and Regulatory
| DEHP is not part of the Material Formulation | DEHP is not part of the material formulation |
Manufacturing
| Total Shelf Life | 3 Years |
| Legal Manufacturer's Address | Velano Vascular, San Francisco, CA |
Packaging
| Quantity - Each | 1 |
| Quantity - Box | 50 |
| Quantity - Case | 200 |
Product Basic Specification
| Sterilization Method | Gamma |
| Length | Inner Tube Length 5.85 inches |
| Material | Inner Tube Material-Polyimide |
| Color | Blue |
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
The PIVO™ Pro Needle-free Blood Collection Device advances a flexible flow tube through the IV catheter to access optimal blood draw conditions, overcoming traditional hurdles to collect high-quality, reliable blood samples.11
The BD Peripheral Line Draw Solution is fully compatible with the BD portfolio of blood collection products and peripheral IV catheters, including BD Vacutainer® Luer-Lok™ Access Device, BD Vacutainer® Blood Collection Tubes, Nexiva™ Closed Catheter System with NearPortTM IV Access, AccuCath Ace™ Intravascular Catheter as well as the BD Insyte™ Autoguard™ Safety IV Catheter and BD Cathena™ Safety IV Catheter portfolio of products.
The PIVO™ Pro Blood Collection Device provides compassionate care that alleviates fear and anxiety associated with repetitive needlesticks by reducing IV replacement2,13 and using existing access for blood draws11 with minimal disruption to a patient's sleep and healing process.14-16
Compared with venipuncture, patients who received daily phlebotomy collections rated their overall experience a 9.1 out of 10 using the PIVO™ Blood Collection Device.13
The BD Peripheral Line Draw Solution includes the PIVO™ Pro Blood Collection Device and Nexiva™ Closed IV Catheter System with NearPort™ IV Access. Together these products help to reduce the number of sticks associated with venipuncture and IV replacement2 helping to preserve vessel health for future access.4 Reliable peripheral line draws may also provide an alternative to the reliance and associated risks of central line draws.19
Streamline clinical workflow with a innovative line draw solution that optimizes IV performance to effectively perform reliable blood draws and reduce IV replacements, empowering clinician confidence at each patient encounter.2,5
Performing a needle-free blood draw and reducing the number of sticks associated with sample recollections and IV replacements reduces the potential risk of an accidental needlestick injury.2
It preserves your patient’s vessel health4 by maximizing first stick success,18 minimizing IV replacements, and using existing access for blood draws.2,11
Needle phobia terminology differs throughout literature and includes terms that are often used interchangeably: scared of needles, fear of needles, needle fear, Blood-Injection Injury (BII) phobia, fear of injections, and needle anxiety.
If your patients have needle phobia, it could lead to avoidance behavior. This includes exacerbated avoidance of treatment, blood donations, and vaccinations – all which can negatively impact patient care.20,21
The most common device-related approach for addressing needle phobia is utilizing innovations that include smaller/thinner needles, followed by needle-free options.22,23
*Clinical studies were done on previous generations of the PIVO™ Blood Collection Device and Nexiva™ Catheter System. PIVO™ Pro and Nexiva™ with NearPort™ IV Access are the next generations of their respective product families.
If you are a patient or end user, you can contact us yourself, or you may have your caregiver or your physician do that for you. To help us process your
information quickly and effectively, please contact our customer complaints
team.
To better facilitate our investigation, please include the following information in your reporting:
- Product Name and/or Catalog Number
- Lot Number or Serial Number
- Any injuries and/or Harm?
- What is the issue you experienced?
- Is the actual sample or sample representative available? (If possible, please send affected sample)
- Contact name and phone number








