Services
BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.

BD Expands Its Services Capabilities With ZebraSci
ZebraSci brings a rich history rooted in vision inspection, equipment design, and automation now applied to primary containers and complex devices.
RIGHT SOLUTIONS, RIGHT TIME
No matter where you are in the process of combination product development, BD can help you reach your goals.
Efficient services to develop, de-risk
and accelerate delivery of your product
Each of our services is delivered intentionally and carefully to de-risk your development journey and accelerate the launch of your drug combination product on the market
-

Flexible offer
Our goal : offer services tailored to your needs
-

Access to scientific testing experts
Our goal : provide rapid and accurate answers to your questions
-

World-class container selection and validation
Our goal : develop optimal solutions for your primary container and secondary device
-

Combination product knowledge and testing capabilities
Our goal : ensure component and system performance consistency
-

Customizable protocols
Our goal : deliver relevant results addressing your specific needs
-

GMP-compliant labs with state-of-the-art equipment
Our goal : provide you with high-quality test results that can be leveraged in regulatory filings
Develop. De-risk. Deliver.
BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.
Supporting pharmaceutical organizations in the development process with:
- Fast access to low quantities** of drug delivery system components for development stage work to help support flexibility and streamline your device selection process.
- Identification of recommended primary containers and secondary injection support devices for a drug's delivery requirements based on BD expertise in drug delivery solutions.
- Proactive delivery of documentation to support combination product development.
- Support of technical problem solving, in primary container and secondary injection support devices and combination product development.
- Research and development data§ for BD PartnerPath™ Program delivery systems to help inform and de-risk combination product development.
*Available for select products and product systems
**Minimum purchase quantity at the lowest sterilized package configuration
§ Data and services are available for specific BD products and product configurations
Consultation services that provide fast access to a breadth of expertise in combination product development via virtual or in-person sessions, including:
- Guidance on country-specific** regulations to strive towards a successful submission
- Key considerations to support drug delivery system selection and process development
- Recommended testing strategies§ to de-risk development activities
- Additional access to relevant combination product development and regulatory submission experience
Accelerated training on BD offerings via virtual / face-to-face sessions
- Expedited access to product information and specifications
- Product specific sessions aimed to support combination product development
*Available for select products and product systems
**Select countries available
§Testing strategy recommendations are non-exhaustive
Expand your reach with regulatory support*
Canada
- BD can provide you with a Letter of Authorization to BD Canada DMF. Make your online request here.
- In support of your DEL (Drug Establishment License), BD will submit to Health Canada a GMP Clearance dossier for foreign manufacturers for BD and BD-subcontracted
sterilization sites.
China
BD can provide you with a Letter of Authorization (LoA) to applicable BD China DMF in the scope of co-evaluation of BD drug packaging material with your combination products.
Europe
BD can support your preparation of a Global Safety and Performance Requirements (GSPR) dossier for submission to an EU Notified Body (NB) in view of obtaining a Notified Body Opinion in line with article 117 of the Medical Device Regulation (MDR).
Japan
BD can support your registration of a combination product to the Pharmaceuticals and Medical Devices Agency (PMDA) by providing a package of documents in line with the Japanese Essential Requirements Checklist (ERCL) following the applicable Ministry of Health, Labour and Welfare Notification.
Korea
BD can support your registration of a combination product by providing a Korean Technical Document in line with Korean guidelines on examining documents for sterilized needles included in drugs, etc. and Korean standards.
Malaysia
BD can support your preparation, in line with the Malaysian guideline on drug-device combination products, of an application to the Malaysian Medical Device Authority (MDA) to obtain an endorsement letter of ancillary component for registration of your combination product.
United States of America
BD can provide you with a Letter of Authorization to BD US DMF. Make your online request here.
*Available for select products and product systems
**Full offerings not shown
Access to GMP production filling assembling, and labeling for not-forhuman-use small volumes, with services including:*§
Filling
• Syringe filling in a clean atmosphere area
• Glass and plastic syringes 1 mL to 50 mL
• Terminal steam sterilization capable
Assembling
• Assembling includes pre-filled syringes with autoinjectors or needle shielding systems
Labeling as per customer input
• Labeling includes syringes and autoinjectors
*Available for select products and product systems
§ Customer samples required
We offer numerous primary container and device characterization services such as:
- Material & mechanical behaviors modelling
- Functional dimension analysis
- Various functional testing on customized systems to understand changes in performance over time
- Predictive PFS/drug analytical studies
- Syringe barrel & stopper performance with drug product
- Autoinjector Powerpak simulations with primary container and drug product
These studies yield data to help clients make informed decisions on primary containers and devices
Evaluate primary container and drug interaction
We provide predictive tools, subvisible particle analysis, Tungsten studies, and extractable and leachable studies to help:
- Support primary container choice
- Assess incompatibilities with tungsten, metal ion and organic leachable
- Assess Interface incompatibilities (Drug degradation/alteration of the device functionality)
- Assess what compounds associated with primary container could be extracted into the drug product
- Evaluate combination product performance
To assist in evaluating device and drug combination product functional performance for needle-based injection systems, safety systems, and autoinjector systems, we offer: - Dose accuracy testing
- Injection time (in air)
- Activation and glide force testing
- Additional testing capabilities
- Assess closure integrity of the system
- We can assist in developing a holistic approach to your container closure integrity strategy for quality assurance throughout the entire product lifecycle.
We offer several methods:
- High Voltage Leak Detection
- Helium Leak Detection
- Vacuum Decay Detection
- Dye Leak Detection
*Available for select products and product systems
Our experienced team assists in developing a robust and rapid complaint handling process.
We help device teams conserve precious resources in developing and maintaining product complaint programs as well as reduce the pains of surge work
We can support customers with their market complaint management programs. We receive complaint samples, decontaminate them and execute a protocol to determine root cause of the complaint using methods such as:
- Dimension & silicone measurement
- FM/Particles visual
- SubVP evaluation / MFI and HIAC methods
- Functional & performance testings
We can support manufacturing and quality organizations with incoming inspection testing of raw materials
Incoming inspection testing can be related to mechanical performance testing, lubrication inspection, dimension measurements, or other customer requested inspections
We provide lot release testing services for primary containers and devices
- Typical lot release testing can include functional device performance testing or container closure integrity testing (CCIT)
- Lot release testing can support clients when their contract manufacturer organisation (CMO) or contract packager organisation (CPO) may not be as familiar with combination product test methods
Explore our products
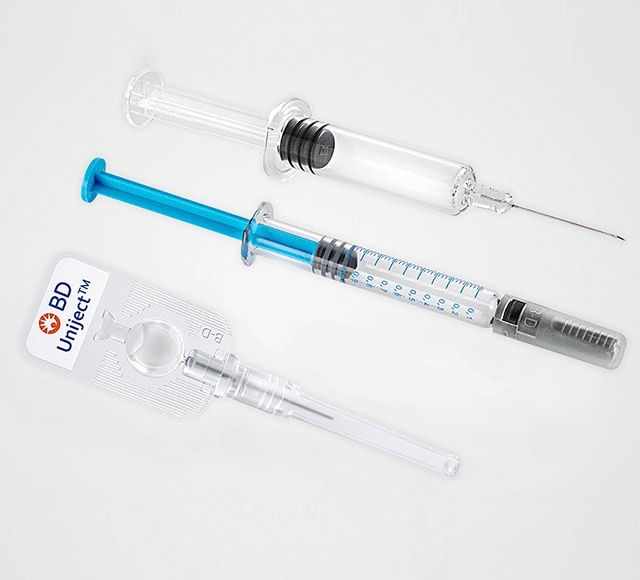
Prefillable syringe systems
BD is uniquely positioned to offer prefillable syringe systems with expertise in drug container interactions, primary container selection and container/device integration for various drug therapies.
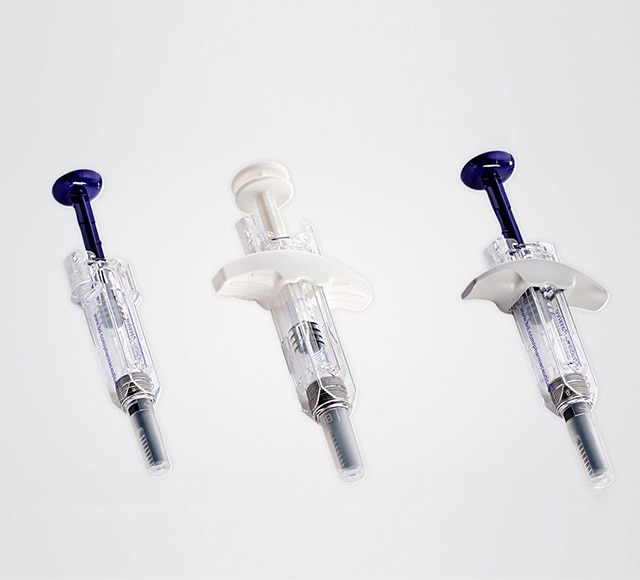
Safety and shielding systems
BD offers a wide range of safety and shielding systems that feature innovative needle shielding system technology for your injectable drug.
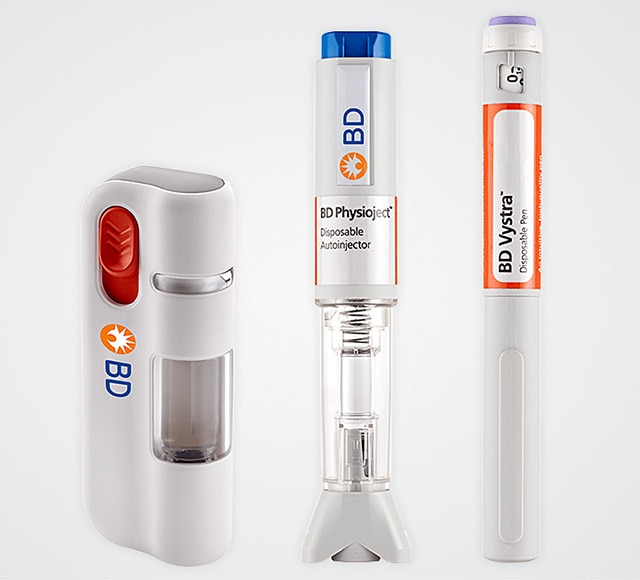
Self injection systems
BD partners with you to develop self-injection systems that enable drug administration across a range of volumes and viscosities, leveraging BD primary container technologies and expertise with a focus on reaching the market faster.

Plunger Stoppers
With our two stopper processing sites, we aim to offer security of supply and business continuity for your drug combination product needs.

Backstops and plunger rods
As part of our integrated approach to help you develop drug combination products, we offer BD Backstops and BD Plunger Rods in a range of sizes, materials and colors.