System Integration
Effective System Integration Streamlines Combination Product Development.

BD Approaches to System Integration: Enabling System Integration
Working with multiple suppliers can be time-consuming, and you must act as the Systems Integrator.
How do you avoid the problems that can arise from working with multiple suppliers and with systems that are not fully integrated?
The answer is simple: work with BD as your single supplier as a trusted partner.
To support the development of your drug delivery system, our cross-functional team of technical, development, regulatory affairs, quality and medical experts offer advice on:
-

Combination of subsystems Optimal
-

Regulation of combination products
-

Minimization of manufacturing risk
-

Supply chain efficiency
-

Traceability
-

Lifecycle management
Explore our products
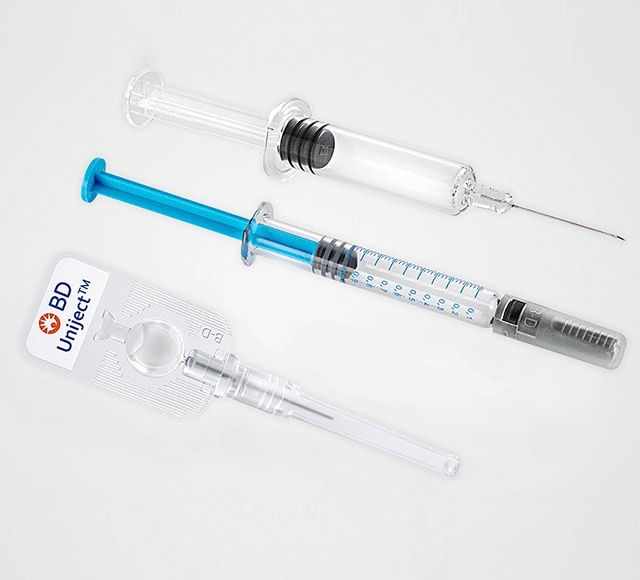
Prefillable syringe systems
BD is uniquely positioned to offer prefillable syringe systems with expertise in drug container interactions, primary container selection and container/device integration for various drug therapies.
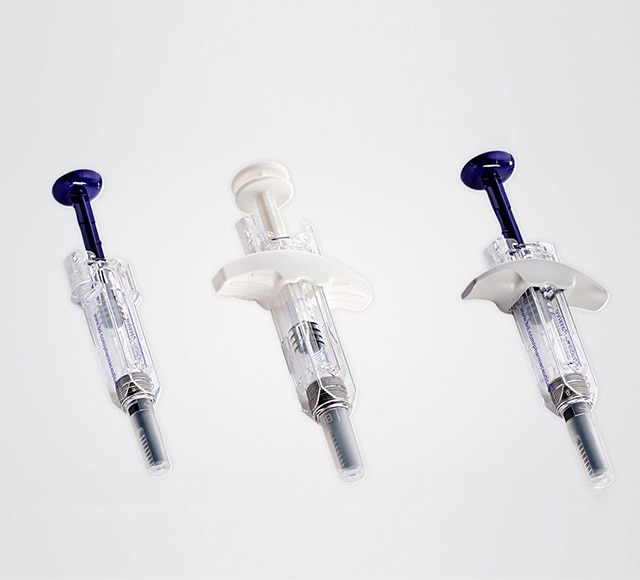
Safety and shielding systems
BD offers a wide range of safety and shielding systems that feature innovative needle shielding system technology for your injectable drug.
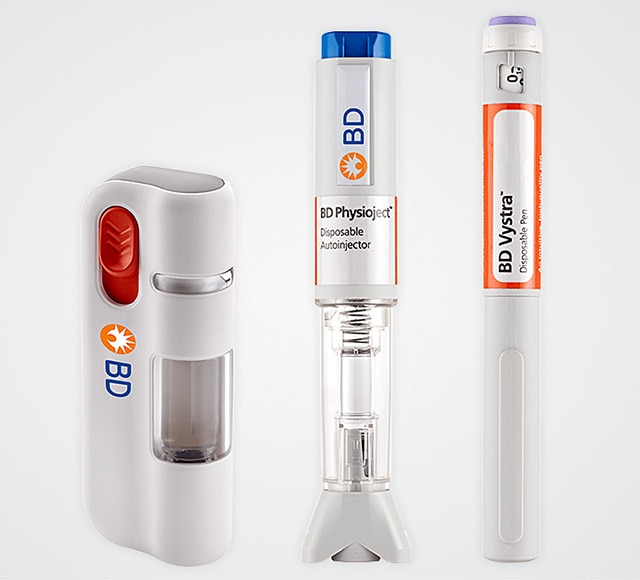
Self injection systems
BD partners with you to develop self-injection systems that enable drug administration across a range of volumes and viscosities, leveraging BD primary container technologies and expertise with a focus on reaching the market faster.

Plunger Stoppers
With our two stopper processing sites, we aim to offer security of supply and business continuity for your drug combination product needs.

Backstops and plunger rods
As part of our integrated approach to help you develop drug combination products, we offer BD Backstops and BD Plunger Rods in a range of sizes, materials and colors.
- Analysis of BD sales from 2014 to 2018 [internal analysis]. Pont-de-Claix, FR: Becton Dickinson and Company; 2019
- Physioject™ post market surveillance quality statement [internal report]. Pont-de-Claix, FR: Becton, Dickinson and company; 2015
- BD External R&D Document: Design Control Evidence Intevia™ 1ml: PIR Number: 2014-0137 Document reference: ERD20200058
- BD design control procedure 103.107.350GP WW Product Design Control [internal document], Franklin Lakes, NJ, USA
- Market Research, “Challenges with System Integration" [external study], Franklin Lakes, NJ, USA: GLG 2019
- Zhaoyang Li & Rachael Easton (2018) Practical considerations in clinical strategy to support the development of injectable drug-device combination products for biologics, mAbs, 10:1, 18-33, DOI: 10.1080/19420862.2017.1392424 - ref-16128
- DeGrazio F, Paskiet D. Injectable Combination Product Development: Facilitating Risk-Based Assessments for Efficiency and Patient Centric Outcomes. J Pharm Sci. 2020 Jul;109(7):2101-2115. doi: 10.1016/j.xphs.2020.03.020. Epub 2020 Apr 6. PMID: 32272133 - ref-17453