| SYMBOL | STANDARD REFERENCE | STANDARD TITLE | SYMBOL TITLE | EXPLANATORY TEXT |
|---|
 | ISO 15223-1: 2021
Reference no. 5.1.1. (ISO 7000-3082) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Manufacturer | Indicates the medical device manufacturer |
 | ISO 15223-1: 2021
Reference no. 5.1.2 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Authorized Representative in the European Community/ European Union | Indicates the authorized representative in the European Community / European Union |
 | ISO 15223-1: 2021
Reference no. 5.1.3. (ISO 7000-2497) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Date of manufacture | Indicates the date when the medical device was manufactured |
 | ISO 15223-1: 2021
Reference no. 5.1.4. (ISO 7000-2607) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Use-by date
Use by date | Indicates the date after which the medical device is not to be used
iso_15223 Use-by date
iso_grs_7000_2607 Use by date |
 | ISO 15223-1: 2021
Reference no. 5.1.5. (ISO 7000-2492) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Batch code | Indicates the manufacturer's batch code so that the batch or lot can be identified. Synonyms for “batch code” are “lot number”, “lot code” and “batch number”. |
 | ISO 15223-1: 2021
Reference no. 5.1.6. (ISO 7000-2493) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Catalogue number
Catalog number | Indicates the manufacturer's catalog number so that the medical device can be identified
ISO 15223 Catalogue number
ISO 7000 Catalog number |
 | ISO 15223-1: 2021
Reference no. 5.1.7. (ISO 7000-2498) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Serial number | Indicates the manufacturer's serial number so that a specific medical device can be identified |
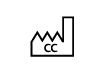 | ISO 15223- 1:2021
Reference no. 5.1.11. (IEC 60417-6049) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Country of manufacture | To identify the country of manufacture of products |
 | ISO 15223-1: 2021
Reference no. 5.2.1. (ISO 7000-2499) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterile | Indicates a medical device that has been subjected to a sterilization process |
 | ISO 15223-1: 2021
Reference no. 5.2.2. (ISO 7000-2500) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterilized using aseptic processing techniques | Indicates a medical device that has been manufactured using accepted aseptic techniques |
 | ISO 15223-1:2021
Reference no. 5.2.3. (ISO 7000-2501) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. |
 | ISO 15223-1:2021
Reference no. 5.2.4. (ISO 7000-2502) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation |
 | ISO 15223-1:2021
Reference no. 5.2.5. (ISO 7000-2503) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterilized using steam or dry heat | To indicate that the device is provided sterile and has been sterilized using steam or dry heat |
 | ISO 15223-1:2021
Reference no. 5.2.6.(ISO 7000- 2608) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Do not resterilize | Indicates a medical device that is not to be resterilized |
 | ISO 15223-1: 2021
Reference no. 5.2.7. (ISO 7000-2609) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Non-sterile | Indicates a medical device that has not been subjected to a sterilization process |
 | ISO 15223-1: 2021
Reference no. 5.2.9. (ISO 7000-3084) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterile fluid path | Indicates the presence of a sterile fluid path within the medical device in cases when other parts of the medical device, including the exterior, might not be supplied sterile |
 | ISO 15223-1: 2021
Reference no. A.13, NOTE 1 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Example of sterile fluid path | Examples of use of symbol 5.2.9 for “Sterile fluid path”. Medical device contains a sterile fluid path that has been sterilized using ethylene oxide. |
 | ISO 15223-1: 2021
Reference no. A.13, NOTE 2 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Example of sterile fluid path | Examples of use of symbol 5.2.9 for “Sterile fluid path”. Medical device contains a sterile fluid path that has been sterilized using irradiation. |
 | ISO 15223-1: 2021
Reference no. 5.3.1. (ISO 7000-0621) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Fragile, handle with care | Indicates a medical device that can be broken or damaged if not handled carefully |
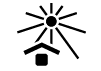 | ISO 15223-1: 2021
Reference no. 5.3.2. (ISO 7000-0624) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Keep away from sunlight | Indicates a medical device that needs protection from light sources |
 | ISO 15223-1: 2021
Reference no. 5.3.4. (ISO 7000-0626) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Keep dry
Keep away from rain | Indicates a medical device that needs protection from moisture
ISO 15223 Keep dry
ISO 7000 Keep away from rain |
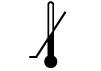 | ISO 15223-1: 2021
Reference no. 5.3.5. (ISO 7000-0534) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Lower limit of temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed |
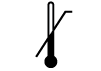 | ISO 15223-1: 2021
Reference no. 5.3.6. (ISO 7000-0533) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed |
 | ISO 15223-1: 2021
Reference no. 5.3.7. (ISO 7000-0632) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed |
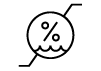 | ISO 15223-1: 2021
Reference no. 5.3.8. (ISO 7000-2620) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Humidity limitation | Indicates the range of humidity t which the medical device can be safely exposed |
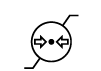 | ISO 15223- 1:2021 Reference no. 5.3.9 (ISO 7000-2621) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Atmospheric pressure limitation
Atmospheric Pressure limitation | To indicate the acceptable upper and lower limits of atmospheric pressure for transport and storage.
ISO 15223 Atmospheric pressure limitation
ISO 7000 Atmospheric Pressure limitation |
 | ISO 15223-1: 2021
Reference no. 5.2.8. (ISO 7000-2606) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Do not use if package is damaged and consult instructions for use | Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information |
 | ISO 15223-1:2021 Reference no. 5.4.1. (ISO 7000-0659) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Biological risks | Indicates that there are potential biological risks associated with the medical device |
 | ISO 15223-1:2021
Reference no. 5.4.2. (ISO 7000- 1051) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Do not re-use | Indicates a medical device that is intended for one single use only
NOTE: Synonyms for “Do not reuse” are “single use” and “use only once”. |
 | ISO 15223-1:2021 Reference no. 5.4.3. (ISO 7000-1641)
| Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Consult instructions for use or consult electronic instructions for use | Indicates the need for the user to consult the instructions for use
iso_15223 Consult instructions for use
iso_grs_7000_1641 Operator's manual; operating instructions |
 | ISO 15223-1:2021
Reference no. A.16 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Consult instructions for use or consult electronic instructions for use | Example of use of symbol 5.4.3, “Consult instructions for use or consult electronic instructions for use” for an electronic instruction for use (eIFU) |
 | ISO 15223-1: 2021 Reference no. 5.4.4. (ISO 7000-0434A) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Caution | To indicate that caution is necessary when operating the device or control close to where the symbol is placed, or to indicate that the current situation needs operator awareness or operator action in order to avoid undesirable consequences |
 | iso_grs_7010_WOO1
| Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | General warning sign | To signify a general warning |
 | ISO 15223-1: 2021
Reference no. 5.4.5. (ISO 7000- 2025) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains or presence of natural rubber latex | Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of a medical device |
 | ISO 15223- 1:2021
Reference no. 5.4.6 (ISO 7000-3701) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains human blood or plasma derivatives | Indicates a medical device contains or incorporates human blood products or plasma derivatives |
 | ISO15223- 1:2021
Reference no. 5.4.7. (ISO 7000-3702) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains a medicinal substance | Indicates a medical device that contains or incorporates a medicinal substance |
 | ISO 15223- 1:2021
Reference no. 5.4.8. (ISO 7000-3699) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains biological material of animal origin | Indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin |
 | ISO 15223- 1:2021
Reference no. 5.4.9. (ISO 7000-3700) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains biological material of human origin | Indicates a medical device that contains biological tissue, cells, or their derivatives, of human origin |
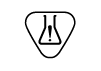 | ISO15223- 1:2021
Reference no. 5.4.10. (ISO 7000-3723) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains hazardous substances | Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine-disrupting properties |
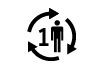 | ISO15223- 1:2021
Reference no. 5.4.12. (ISO 7000-3706) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Single patient-multiple use | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient |
 | ISO 15223-1:2021
Reference no. 5.5.1. | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | In Vitro diagnostic medical device | Indicates a medical device that is intended to be used as an in vitro diagnostic medical device |
 | ISO 15223-1: 2021
Reference no. 5.5.2. | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Control | Indicates a control material that is intended to verify the performance of another medical device |
 | ISO 15223-1: 2021
Reference no. 5.5.3. (ISO 7000-2495) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Negative control | Indicates a control material that is intended to verify the results in the expected negative range |
 | ISO 15223-1: 2021
Reference no. 5.5.4. (ISO 7000-2496) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Positive control | Indicates a control material that is intended to verify the results in the expected positive range |
 | ISO 15223-1: 2021
Reference no. 5.5.5. (ISO 7000-0518) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains sufficient for <n> tests | Indicates the total number of IVD tests that can be performed with the IVD medical device. |
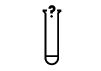 | ISO 15223-1: 2021
Reference no. 5.5.6. (ISO 7000-3083) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | For IVD performance evaluation only | Indicates an IVD device that is intended to be used only for evaluating its performance characteristics before it is placed on the market for medical diagnostic use |
 | ISO 15223-1: 2021
Reference no. 5.6.2. (ISO 7000-2722) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Fluid path | Indicates the presence of a fluid path |
 | ISO 15223-1: 2021
Reference no. 5.6.3. (ISO 7000-2724) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Non-pyrogenic | Indicates a medical device that is non-pyrogenic |
 | ISO 7000
Reference no. 2723 | Graphic symbols for use on electrical equipment | Non-pyrogenic fluid
path | On medical devices: to indicate that the fluid path is non-pyrogenic |
 | ISO 15223-1:2021
Reference no. 5.6.4. (ISO 7000-2726) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Drops per milliliter
Drops per millilitre | On medical devices: to indicate the number of drops per milliliter. That means the design of the drip tube in the drip chamber of the system.
iso_grs_7000_2726 Drops per milliliter
iso_154223 Drops per millilitre |
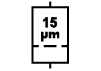 | ISO 15223-1: 2021
Reference no. 5.6.5. (ISO 7000-2727) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Liquid filter with pore size | Indicates an infusion or transfusion
system of the medical device that
contains a filter of a particular
nominal pore size |
 | ISO 15223-1: 2021
Reference no. 5.7.1. (ISO 7000-2610) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Patient number | Indicates a unique number associated with an individual patient |
 | ISO15223- 1:2021
Reference no. 5.7.3 (IEC 60417-5664) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Patient identification | Indicates the identification data of the patient |
 | ISO 15223- 1:2021
Reference no. 5.7.4 (ISO 7000-3705) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Patient information website | Indicates a website where a patient may obtain additional information on the medical product |
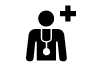 | ISO/DIS 15223-1:2021
Reference no. 5.7.5. (ISO 7001-PI PF 044) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Health care center or doctor | To indicate the address of the health care center or doctor where medical information about the patient may be found |
 | ISO15223- 1:2021
Reference no. 5.7.6 (IEC 60417-5662) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Date | To identify the date that information was entered, or a medical procedure took place |
 | ISO/DIS 15223- 1:2021
Reference no. 5.7.7 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Medical device | Indicates the item is a medical device |
 | ISO 15223-1:2021
Reference no. 5.7.8. (ISO 7000-3728) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Translation | To identify that the original medical device information has undergone a translation which supplements or replaces the original information |
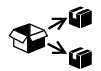 | ISO 15223-1:2021
Reference no. 5.7.9. (ISO 7000-3727) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Repackaging | To identify that a modification to the original medical device packaging configuration has occurred |
 | IEC 60601- 1
Reference no. Table D1, Symbol 8 (IEC 60417-5032) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | Alternating current | To indicate on the rating plate
that the equipment is suitable for
alternating current only; to identify
relevant terminals |
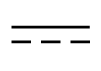 | IEC 60601-1
Reference no. Table D.1, Symbol 4 (IEC 60417- 5031) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | Direct current | To indicate on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals |
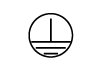 | IEC 60601-1
Reference no. Table D.1, Symbol 6 (IEC 60417- 5019) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance. | Protective earth; protective ground | To identify any terminal which is intended for connection to an external conductor for protection against electric shock in case of a fault, or the terminal of a protective earth (ground) electrode |
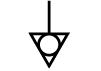 | ICE 60601- 1
Reference no. Table D1, Symbol 8 (IEC 60417-5021) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | Equipotentiality | To identify the terminals which, when
connected together, bring the various
parts of equipment or of a system
to the same potential, not necessarily
being the earth (ground) potential,
e.g. for local bonding |
 | IEC 60417
Reference no. Table D.1, Symbol 9 (IEC 60417- 5172) | Graphic symbols for use on electrical equipment | Class II equipment | To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 61140 |
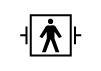
 | IEC 60601-1, Reference no. Table D.1, Symbol 19 (ICE 60417-5480) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | TYPE B APPLIED PART | N/A |
 | IEC 60601-1, Reference no. Table D.2, Symbol 20 (ICE 60417-5333) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | TYPE BF APPLIED PART | To identify a type BF applied part complying with IEC 60601-1 |
 | IEC 60601-1 Reference no. Table D.1, Symbol 21 (IEC 60417-5335) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance. | Type CF applied part. | To identify a type CF applied part complying with IEC 60601-1 |
 | IEC 60601-1 Reference no. Table D.1, Symbol 26 (IEC 60417-5334) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance. | Defibrillation-proof Type BF applied part | To identify a defibrillation-proof type BF applied part complying with IEC 60601-1 |
 | IEC 60601-1 Reference no. Table D.1, Symbol 21 (IEC 60417-5336) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance. | Defibrillation-proof Type CF applied part | To identify a defibrillation-proof Type CF applied part complying with IEC 60601-1 |
 | IEC 60601-1 Database Reference no. Table D2, Safety sign 5 (ISO 7010-P017) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance. | No pushing | To prohibit pushing against an object |
 | IEC 60601-1, Reference no. Table D.2, Safety sign 10 (ISO 7010-M002) | Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance | Refer to instruction manual/booklet | To signify that the instruction manual/booklet must be read |
 | IEC 60601-1 (IEC 60529) Table D.3; Code 2 6.3; Table D.3; Code 2 | Medical electrical equipment – Part 1:General requirements. for basic safety and essential performance | Degree of protection | N1 = 0 Non-protected; 1 Protected against solid foreign objects of 50 mm Ø and greater; 2 Protected against solid foreign objects of 12,5 mm Ø and greater; 3 Protected against solid foreign objects of 2,5 mm Ø and greater; 4 Protected against solid foreign objects of 1,0 mm Ø and greater; 5 Dust-protected; 6 Dust-tight N2 = 0 Non-protected; 1 Protection against vertically falling water drops; 2 Protection against vertically falling water drops when ENCLOSURE tilted up to 15°; 3 Protected against spraying water; 4 Protected against splashing water; 5 Protected against water jets; 6 Protected against powerful water jets; 7 Protected against the effects of temporary immersion in water; 8 Protected against the effects of continuous immersion in water |
 | IEC 60601-1, (IEC 60529) Reference no. 6.3; Table D.3, Code 2 | Medical electrical equipment – Part 1:General requirements. for basic safety and essential performance | Degree of protection | IP22: N1=2, Protected against solid foreign objects of 12,5 mm Ø and greater; N2=2, Protection against vertically falling water drops when ENCLOSURE tilted up to 15° |
 | IEC 60601-1, (IEC 60529) Reference no. 6.3; Table D.3, Code 2 (Refer to IEC 60529; see 7.2.9 and 11.6.5) | Medical electrical equipment – Part 1:General requirements. for basic safety and essential performance | Degree of protection | IP27: N1=2, Protected against solid foreign objects of 12,5 mm Ø and greater; N2=7, Protected against the effects of temporary immersion in water |
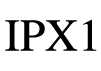 | IEC 60601-1 (IEC 60529) Reference no. 6.3; Table D.3; Code 2
| Medical electrical equipment – Part 1: General requirements. for basic safety and essential performance | Degree of protection | IPX1: N1=X, which means it was not required; N2=1, Protection against vertically falling water drops |
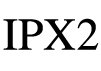 | IEC 60601-1 (IEC 60529) Reference no. 6.3, Table D.3, Row 2 | Medical electrical equipment – Part 1: General requirements. for basic safety and essential performance | Degree of protection | IPX2: N1=X, which means it was not required; N2=2, Protection against vertically falling water drops when ENCLOSURE tilted up to 15° |
 | IEC 60601-1 (IEC 60529)
Reference no. Table 6.3; D.3, Code 2 | Medical electrical equipment – Part 1: Part 1: General requirements. for basic safety and essential performance | Degree of protection | IP33: N1=3, Protected against solid foreign objects of 2,5 mm Ø and greater; N2=3, Protected against spraying water |
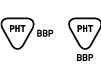 | BS EN 15986:2011
Reference no. A.4 | Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates | Contains or presence of phthalate: benzyl butyl phthalate (BIPingreBP) | Medical device is derived from or manufactured from products containing phthalate: benzyl butyl phthalate (BBP) |
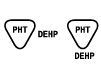 | BS EN 15986:2011
Reference no. A.4 | Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates | Contains or presence of phthalate: bis (2- ethylhexyl) phthalate (DEHP) | Medical device is derived from or manufactured from products containing phthalate: bis (2- ethylhexyl) phthalate (DEHP) |
 | BS EN 15986:2011
Reference no. A.5 | Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates | Contains or presence of phthalate: combination of bis (2-ethylhexyl) phthalate (DEHP) and benzyl butyl phthalate (BBP) | Medical device is derived from or manufactured from products containing bis (2-ethylhexyl) phthalate (DEHP) and benzyl butyl phthalate (BBP) |
 | BS EN 15986
Annex B | Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates. | Negation symbol plus Presence of phthalate symbol, together meaning free of phthalates | Manufacturers wishing to communicate the meaning “does not” or “is not” where a symbol expressing this meaning does not exist, should follow the method set out in EN 80416- 3:2002, Clause 7 |
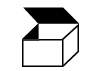 | ISO 7000
Reference no. 2794 | Graphical symbols for use on equipment. | Packaging unit | To indicate the number of pieces in the package |
 | ISO 7000
Reference no. 0623 | Graphical symbols for use on equipment - registered symbols | This way up | N/A |
 | ISO 7000
Reference no. 2402 | Graphical symbols for use on equipment-Registered symbols | Do not stack | To indicate that the item shall not be vertically stacked, either because of the nature of the transport packaging or because of the nature of the items themselves |
 | ISO 7000
Reference no. 1056 | Graphical symbols for electrical equipment in medical practice - Registered symbols | Oil; fluid | To identify oil or other non-water base fluid. On an indicator to identify oil or used to identify a fill cap |
 | ISO 7001
Reference no. PI PF 017 | Graphical symbols - Public information symbols | Telephone | To indicate the location of public telephone |
 | ISO 7001
Reference no. PI PF 002 Hospital | Graphical symbols - Public information symbols | Hospital | Indicates the location of a hospital |
 | ISO 7000_3079
Reference no. 3079 | Graphical symbols for use on equipment - registered symbols | Open here | To identify the location where the package can be opened and to indicate the method of opening it.
|
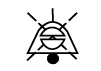 | IEC 60417-1
Reference no. ISO 7000-5576-3 | Graphical symbols for Use on Equipment | Bell, cancel temporary acknowledged; temporary acknowledged | To identify the control whereby a bell may be temporarily acknowledged or to indicate that the bell has been temporarily acknowledged |
 | IEC 60417-1
Reference no. ISO-7000-5576-2 | Graphical symbols for use on equipment | Bell, cancel temporary | To indicate the operating status of the bell being temporarily canceled |
 | IEC 60417-1
Reference no. 5850 | Graphic symbols for use on electrical equipment | Serial interface | To identify on a connector for a serial data connection |
 | IEC 60417-1
ISO 7000-5569
not IEC 60417 Reference no. 5569 | Graphic symbols for use on electrical equipment | Locking, general | To identify on a control that a function is locked or to show the locked status |
 | IEC 60417-1
Reference no. 5570 | Graphic symbols for use on electrical equipment | Unlocking | To identify on a control that a function is not locked or to show the unlocked status |
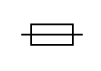 | IEC 60417-1 Reference no. ISO 7000-5016 | Graphical symbols for use on equipment | Fuse | To identify fuse boxes or their location |
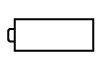 | IEC 60417
Reference no. ISO 7000-5001B | Graphic symbols for use on electrical equipment | Battery, general | On battery powered equipment |
 | IEC 60417-1 Reference no. ISO 7000-6042 | Graphical symbols for use on equipment | Caution, risk of electrical shock | To identify equipment, for example, the welding power source, that has risk of electrical shock |
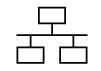 | IEC 60417-1 Reference no. ISO 7000-5988 | Graphical symbols for use on equipment | Computer network | To identify the computer network itself or to indicate the connecting terminals of the computer network |
 | IEC 60417
Reference no. 7000-5140 | Graphical Symbols for Use on Equipment | Non-ionizing electromagnetic radiation | N/A |
 | IEC 60417
Reference no. 5668 | Graphical Symbols for Use on Equipment | Nurse | To indicate a reference to a nurse or the nursing staff, e.g. on a call button |
 | ISO 15223-1:2021Reference no. "5.1.10(IEC 60417-6050)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Model number | To identify the model number or type number of a product. In the application of this symbol, the model number or type number of the product should be accompanied with this symbol |
 | IEC 60417
Reference no. 5845 | Graphical Symbols for Use on Equipment | Inner diameter | To indicate a reference to the inner diameter |
 | IEC 60417
Reference no. 5846 | Graphical Symbols for Use on Equipment | Outer diameter | To indicate a reference to the outer diameter |
 | ASTM F2503
Reference no. ASTM F2503; Table 2; 7.4.6.1; Fig. 6, 7 | Standard practice for Making Medical Devices and other item for safety in the magnetic resonance environment | Magnetic Resonance (MR) safe | 3.1.13: An item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electricaly nonconductive, nonmetallic, and nonmagnetic. |
 | ASTM F2503
Reference no. Tabel 2; 7.4.6.1; Fig 6,7 | Standard Practice for Marking Medical Devices and Other Items for Safety in the magnetic resonance environment. | MR Conditional | 3.3.1.11: an item with demonstrated safety in the MR environment within defined conditions including conditions for the static magnetic field, the time-varying gradient magnetic fields and the radiofrequency fields. |
 | ASTM F2503
Reference no. Table 2, Symbol 7.3.3;
7.4.9.1; Fig. 9
| Standard Practice for Marking Medical Devices and other Items for safety in the Magnetic Resonance Environment | (MR) Unsafe | 3.1.14: An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment |
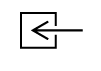 | IEC-TR-60878
Reference no. (ISO 7000-0794) | Graphical symbols for electrical equipment in medical practice | Input: entrance | To identify an entrance, for example exhaust gas entry for measurement (for example of CO- value) |
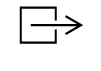 | IEC-TR-60878
Reference no. (ISO 7000-0795) | Graphical symbols for electrical equipment in medical practice | Output; exit | To identify an exit, for example of an hydraulic pump |
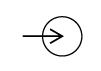 | IEC-TR-60878
Reference no. (ISO 7000-5034) | Graphical symbols for electrical equipment in medical practice | Input | To identify an entrance, for example exhaust gas entry for measurement (for example of CO- value) |
 | IEC-TR-60878
Reference no. (ISO 7000-5134) | Graphical symbols for electrical equipment in medical practice | Electrostatic sensitive devices | To indicate packages containing electrostatic sensitive devices, or to identify a device or a connector that has not been tested for immunity to electrostatic discharge. |
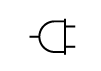 | IEC-TR-60878
Reference no. (ISO 7000-5534) | Graphic symbols for electrical equipment in medical practice | Power Plug | To identify connecting means (e.g. plug or cord) to the power source (mains) or to identify the storage place for the connecting means |
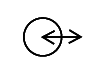 | IEC-TR-60878
Reference no. 1(ISO 7000-5448) | Graphical symbols on equipment | Input/output | To identify a combined input/output connector or mode |
 | IEC-TR-60878
Reference no. ISO 7000-1135 | Graphic symbols for use on electrical equipment in a medical practice | General symbol for
recover/recyclable | To indicate that the marked item or its material is part of a recovery or recycling process |
 | IEC-TR-60878
Reference no. IOS 7000-2403 | Graphic symbols for use on electrical equipment in a medical practice | Stacking limit by number | To indicate that the items shall not be vertically stacked
beyond the specified number, either because of the nature of the transport packaging or because of the nature of the items themselves. |
 | EU 2017-745 EU 2017-746
Reference no. ANNEX V | REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices, amending Directive 2001/83/ EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/ EEC and 93/42/EEC | CE marking | (43) ‘CE marking of conformity’ or ‘CE marking’ means a marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in this Regulation and other applicable Union harmonisation legislation providing for its affixing |
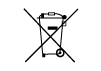 | DIRECTIVE
2012/19/
EU (WEEE) | N/A | Collect separately | Separate collection for waste of electrical and electronic equipment. Do not dispose of battery
in municipal waste. The symbol indicates separate collection for battery is required. |
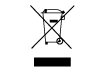 | Directive 2002/96/ EC (repealed). | Replaced by DIRECTIVE 2012/19/EU which does NOT contain this symbol. | Waste stream disposal status | Do not dispose of electronic products in the general waste stream |
 | GHS
Reference no. 1.4.10.4.2.3 A1.7 | Globally Harmonized System of Classification and Labeling of Chemicals (GHS), Eighth Revised Edition | Highly flammable | Medical device contains materials that are highly flammable. Appropriate caution should be taken |
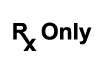 | N/A | N/A | Prescription Use Only | Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |
 | N/A | N/A | Collection time | Time that a specific specimen was collected from the patient |
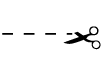 | N/A | N/A | Cut | Directs health care practitioner to cut a package |
 | N/A | N/A | Collection Date | Date that a specific specimen was collected from the patient |
 | N/A | N/A | Keep away from light | Medical device should be shielded or kept away from light sources |
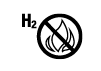 | N/A | N/A | Hydrogen gas is generated | Medical device generates hydrogen gas, caution |
 | N/A | N/A | Perforation | Medical device packaging contains a perforation to aid in opening |
 | N/A | Federal Communications Commission | 21 CFR Part 15 | Meets FCC requirements per 21 CFR Part 15 |
 | EC/94/62 | European Packaging and Packaging Waste Directive | The Green Dot symbol | On packaging, the Green Dot means that for such packaging a financial contribution has been paid to a qualified national packaging recovery organization set up in accordance with the principles defined in European Packaging and Packaging Waste Directive 94/62 and the respective national law. |
 | N/A | N/A | Start panel sequence number | N/A |
 | N/A | N/A | End panel sequence number | N/A |
 | N/A | N/A | Do not freeze | Indicates the medical device should not be frozen |
 | N/A | N/A | Sterile™ Solution (for ChloraPrep use only as of July2020) | For ChloraPrep use only as of July 2020 |
 | N/A | N/A | Russian product
conformity mark | Product compliant with GOST standard(s) |
 | N/A | N/A | Ukrainian conformity mark | Product compliant with Resolution No. 753 |
 | N/A | N/A | UkrSEPRO conformity mark | Product compliant with DSTU (Ukrainian regulatory requirements) |
 | N/A | N/A | Stainless steel instruments of Russian origin | Made in Russia from corrosion-resistant steel |
 | N/A | N/A | Canadian and US Certification mark | Products bearing this mark have been tested and certified in accordance with applicable US and Canadian electrical safety and performance standards |
 | N/A | N/A | Australian Communications Authority | Complies with Australian Communications Requirements |
 | N/A | N/A | USA | Manufactured in the USA and/or applies to medical devices sold in the USA |
 | ISO/DIS 15223- 1:2021
Reference no. 5.2.11. (ISO 7000-3707) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Single sterile barrier system | Indicates a single sterile barrier system |
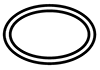 | ISO 15223-1: 2021 Reference no. (ISO 7000-3704)
| Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Double sterile barrier system | Indicates two sterile barrier systems |
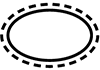 | ISO 15223-1: 2021
Reference no. (ISO 7000-3708) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Single sterile barrier system with protective packaging outside | Indicates a single sterile barrier system with protective packaging outside |
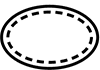 | ISO 15223-1: 2021
Reference no. 5.2.13 (ISO 7000-3709) | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Single sterile barrier system with protective packaging inside | Indicates a single sterile barrier system with protective packaging inside |
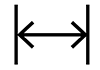 | N/A | N/A | Length | To indicate the approximate length medical device |
 | MedtechEurope.Org | New IVD Regulation EU 2017/746 | Device for self-testing | This symbol indicates that the device is a self-test in vitro diagnostic device. This means that a lay person can use it even without formal healthcare or medical experience |
 | MedtechEurope.Org | New IVD Regulation EU 2017/746 | Device for near-patient testing | This symbol indicates that the device is only to be used in a near patient setting by a health professional. Typically, such tests are used in ambulances, emergency units, patient homes, or workplaces etc. A ‘near patient test’ is not to be used by the patient themselves. |
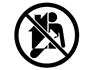 | MedtechEurope.Org | New IVD Regulation EU 2017/746 | Device not for self-testing | This symbol indicates that the device (applies to rapid tests only) is not intended for self-testing. A rapid test with this symbol should only be used by a trained medical or a lab professional in an appropriate setting. Manufacturers may choose to add this symbol next to the ‘’for near patient testing’’ symbol to emphasise the intended user of the test. |
 | MedtechEurope.Org | New IVD Regulation EU 2017/746 | Device not for near-patient testing | This symbol indicates that the device (applies to rapid tests only) is not intended for near-patient testing. A rapid test with this symbol on its label should only be used by a trained laboratory professional in a laboratory. This symbol should be put on rapid tests that are intended for exclusive use in a laboratory environment. |
 | ISO 7010 WO21 | N/A | Warning; Flammable Material | To warn of flammable material. |
 | ISO 7000 ISO 7886-3 | Graphical symbols for use on equipment | Re-use prevention | A feature that allows one use and prevents further uses. |
 | ISO 7000 | N/A | RFID tag, general | On packaging, packaging containers, and equipment: To indicate the presence of the RFID tag incorporated within the packaging, container, or equipment without identifying the specific air interface or data structure employed. |
 | ISO 7001 | Public Information Symbol | Recycling | To indicate the location of a recycling bin or container. |
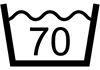 | ISO 7000 | Symbols for labeling instructions for cleansing and care procedures of textiles | Washing, normal process, maximum 70 Celsius | To indicate that cleaning the textile article is allowed using normal washing process at maximum temperature 70 degrees Celsius. |
 | ISO 7000 | Symbols for labeling instructions for cleansing and care procedures of textiles | Bleaching by any agents | To indicate that bleaching the textile article is allowed using any bleaching agents. |
 | ISO 7000 | Symbols for labeling instructions for cleansing and care procedures of textiles | Tumble drying, maximum 60 Celsius | To indicate that the tumble drying process is allowed only with low temperature: exhaust temperature maximum 60 degrees Celsius in the tumble drying process. |
 | ISO 7000 | Symbols for labeling instructions for cleansing and care procedures of textiles | Tumble drying, maximum 80 Celsius | To indicate that the tumble drying process is allowed only with normal temperature: exhaust temperature maximum 80 degrees Celsius in the tumble drying process. |
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Washing, mild process, maximum 60 Celsius | To indicate that cleaning the textile article is allowed using mild washing process at maximum temperature 60 degrees Celsius. |
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Do not tumble dry | To indicate that tumble drying is not allowed in the drying process. |
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Do not iron | To indicate that ironing is not allowed. |
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Do not dry clean | To indicate that dry cleaning is not allowed. |
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Do not bleach | To indicate that bleaching the textile article is not allowed. |
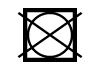
 | ISO 7000 | Symbols for labelling instructions for cleansing and care procedures of textiles | Do not wash | To indicate that washing the textile article is not allowed during the cleaning process. |
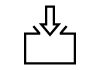 | ISO 7000-2503 | Symbols for labelling instructions for cleansing and care procedures of textiles | Filling | To indicate the filling of a vessel or container by any type of liquid or produce (for example, filling of oil tanks, filling ink reservoirs, filling grain hoppers). |
 | N/A | N/A | Internal sequence number | Internal sequence number |
 | ISO15223-1: 2021 Reference no. 5.7.10 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Unique device identifier | Indicates a carrier that contains unique device identifier information |
 | ISO 15223-1:2021 Reference no."5.1.9(ISO 7000-3724)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Distributor | Indicates the entity distributing the medical device into the locale |
 | ISO 15223-1:2021 Reference no."5.1.8(ISO 7000-3725)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Importer | Indicates the entity importing the medical device into the locale |
 | ISO 7010 | Graphical symbols — Safety colours and safety signs — Registered safety signs | Warning; Laser Beam | To warn of a laser beam |
 |
ISO 15223-1:2021 Reference no. 5.2.10 | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sterilized using vaporized hydrogen peroxide | Indicates a medical device that has been sterilized using vaporized hydrogen peroxide |
 | ISO 15223-1:2021 Reference no."5.3.3(ISO 7000-0615)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Protect from heat and radioactive sources | Indicates a medical device that needs protection from heat and radioactive sources |
 | ISO 15223-1:2021 Reference no."5.4.11(ISO 7000-3703)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Contains nano materials | Indicates a medical device that contains nano materials |
 | ISO 15223-1:2021 Reference no."5.6.1(ISO 7000-2715)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Sampling site | Indicates a medical device or blood processing application that includes a system dedicated to the collection of samples of a given substance stored in the medical device or blood container |
 | ISO 15223-1:2021 Reference no."5.6.6(ISO 7000-2727)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | One-way valve | Indicates a medical device with a valve that allows flow in only one direction |
 | ISO 15223-1:2021 Reference no."5.7.2(ISO 7000-3726)" | Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. | Patient name | Indicates the name of the patient |
 | ISO 20417 Reference no. 6.1.2 d) | Medical devices — Symbols to be used with information to be supplied by
the manufacturer - Part 1: General requirements. | Authorized Representative in Switzerland | Indicates the authorized representative in
Switzerland |
 | ISO 20417 Reference no. 6.1.2 d) | Medical devices — Symbols to be used with information to be supplied by
the manufacturer - Part 1: General requirements. | Authorized Representative in the
United Kingdom | Indicates the authorized representative in
United Kingdom |